BmLid has histone lysine demethylase activity and regulates oogenesis in the silkworm, Bombyx mori
IF 3.7
2区 农林科学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Lepidoptera, a major threat to global agriculture, possesses robust reproductive strategies, making understanding reproduction crucial for developing sterile insect techniques (SIT). The silkworm (Bombyx mori), a model lepidopteran, exhibits polytrophic meroistic oogenesis involving trophocyte-mediated nutrient transport and vitellogenin receptor (VgR)-dependent yolk protein uptake. However, epigenetic regulation of these processes remains unclear. This study investigates the role of BmLid, a histone demethylase, in silkworm oogenesis. Using CRISPR/Cas9, we generated BmLid knockout mutants (ΔBmLid) and confirmed gene disruption via sequencing and western blotting. ΔBmLid females exhibited developmental defects and complete sterility, despite unaffected mating behavior or sperm migration. The H3K4me2/me3 and H3K9me2/me3 levels were significantly increased in ΔBmLid ovaries, indicating BmLid has broad demethylase activity in vivo, too. Transcriptomic analysis showed significant downregulation of VgR, critical for vitellogenin transport, alongside dysregulation of cell junction and apoptosis pathways. These defects disrupted follicular epithelium integrity and yolk protein deposition, mirroring similar phenotypes observed in VgR mutants. Our findings demonstrate that BmLid regulates oogenesis by modulating histone methylation to control VgR expression, cell adhesion, and cell apoptosis, thereby ensuring vitellogenesis. This study highlights epigenetic mechanisms underlying lepidopteran reproduction and identifies BmLid as a potential target for SIT-based pest management strategies.
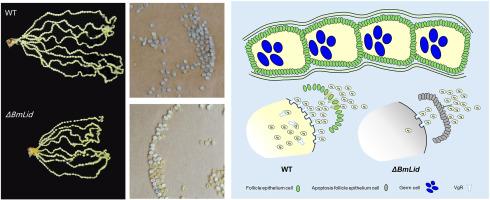
BmLid具有组蛋白赖氨酸去甲基化酶活性,并调节家蚕的卵发生。
鳞翅目是全球农业的主要威胁,具有强大的生殖策略,因此了解生殖对开发昆虫不育技术(SIT)至关重要。家蚕(Bombyx mori)是一种典型的鳞翅目动物,表现出多营养分生发育,涉及滋养细胞介导的营养转运和卵黄蛋白原受体(VgR)依赖的卵黄蛋白摄取。然而,这些过程的表观遗传调控仍不清楚。本研究探讨了组蛋白去甲基化酶BmLid在家蚕卵发生中的作用。使用CRISPR/Cas9,我们产生了BmLid敲除突变体(ΔBmLid),并通过测序和western blotting证实了基因破坏。ΔBmLid雌性表现出发育缺陷和完全不育,尽管未受影响的交配行为或精子迁移。ΔBmLid卵巢中H3K4me2/me3和H3K9me2/me3水平显著升高,表明BmLid在体内也具有广泛的去甲基化酶活性。转录组学分析显示VgR显著下调,这对卵黄蛋白原转运至关重要,同时细胞连接和凋亡途径失调。这些缺陷破坏了卵泡上皮的完整性和蛋黄蛋白沉积,反映了在VgR突变体中观察到的类似表型。我们的研究结果表明,BmLid通过调节组蛋白甲基化来控制VgR的表达、细胞粘附和细胞凋亡,从而确保卵黄形成。这项研究强调了鳞翅目繁殖的表观遗传机制,并确定了BmLid作为基于蚁群的害虫管理策略的潜在目标。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.40
自引率
5.30%
发文量
105
审稿时长
40 days
期刊介绍:
This international journal publishes original contributions and mini-reviews in the fields of insect biochemistry and insect molecular biology. Main areas of interest are neurochemistry, hormone and pheromone biochemistry, enzymes and metabolism, hormone action and gene regulation, gene characterization and structure, pharmacology, immunology and cell and tissue culture. Papers on the biochemistry and molecular biology of other groups of arthropods are published if of general interest to the readership. Technique papers will be considered for publication if they significantly advance the field of insect biochemistry and molecular biology in the opinion of the Editors and Editorial Board.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


