Health-promoting potentials of milk protein hydrolysates: A comparative analysis of camel, cow and donkey resources
Abstract
Background
Milk proteins are a rich source of bioactive peptides with therapeutic properties, including antimicrobial, antioxidant and immunomodulatory effects. Hydrolysis enhances their bioavailability, thereby increasing their potential for treating conditions such as hypertension, inflammation and neurodegenerative diseases. Investigating bioactive peptides derived from milk proteins is essential for developing natural, functional therapeutics that offer fewer side effects than synthetic drugs and provide a sustainable approach to disease prevention and management.
Method
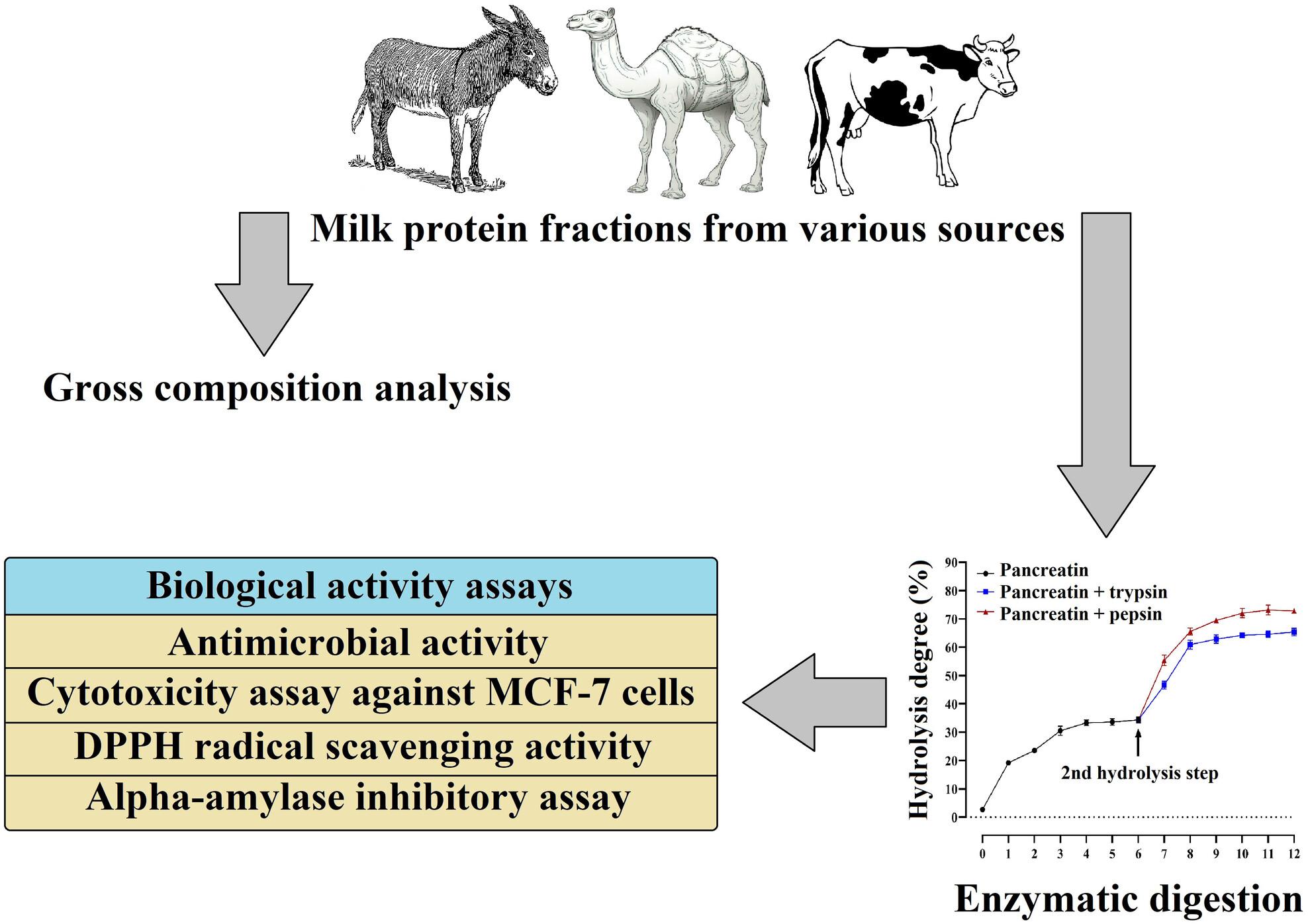
This study examined the composition and health-promoting properties of milk protein hydrolysates derived from camel, cow, and donkey milk using pancreatin alone, pancreatin followed by trypsin and pancreatin followed by pepsin. Casein and whey protein fractions were isolated from each milk type and enzymatically hydrolysed to assess their bioactive potential.
Results
Our results demonstrated a progressive increase in the degree of hydrolysis over time, confirming efficient enzymatic digestion, with the highest values after 12 h under pancreatin–pepsin treatment: 87.74% for camel casein, 80.81% for camel whey, 76.64% for cow whey and 72.81% for donkey casein. The biological findings revealed that donkey milk hydrolysates exhibited superior antimicrobial activity (inhibition zone of 2.1–4.2 cm), cytotoxicity activity against MCF-7 cell line (92.77–99.47% inhibition) and antioxidant activity (33.92–77.52% DPPH inhibition). Furthermore, camel milk hydrolysates showed higher α-amylase inhibitory activity (32.79–93.92%).
Conclusion
These results highlight the potential of donkey milk protein hydrolysates for their antimicrobial, cytotoxicity and antioxidant properties and suggest that camel milk protein hydrolysates may be beneficial for α-amylase inhibition. These hydrolysates hold promise for the development of functional foods and therapeutic agents, offering opportunities for innovation in the food and pharmaceutical industries.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


