Yeast as C1 cell factory: Transforming methanol and Formate into high-value compounds
IF 12.5
1区 工程技术
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
Abstract
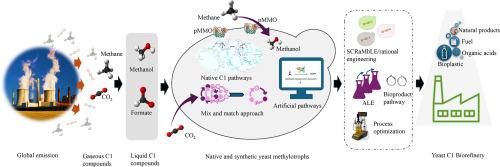
Microbial transformation of greenhouse gases, such as carbon dioxide and methane, into valuable biochemicals appears as a key strategy to sustainably decarbonize manufacturing industries. Numerous unresolved technological constraints still hamper the industrial adoption of these single‑carbon (C1) gas-based bioprocesses. Conversion of these gases into liquid C1 compounds like methanol and formate helps to reduce emissions and close the carbon loop. Certain industrial yeasts possess intrinsic capabilities to tolerate and assimilate methanol and formate, which opens an attractive route to eco-efficiently valorise these compounds. To increase the C1-based biomanufacturing potential of yeasts, synthetic methylotrophy has been developed in versatile non-methylotrophic chassis. Strategic non-rational genome engineering and strain evolutions combined with rational designs brings to light hidden C1-pathways and mechanisms of substrate tolerance. Developments in methanol-based bioproduction include simple organic acids with clear promise for industrial scale-up as well as proof-of-concept investigations of complex polyketides with intricate pathways. Recent advances in bioproduction have demonstrated encouraging results from techniques such as modular co-culture engineering and peroxisomal coupling of biosynthetic pathways with C1 metabolism. Formate-based growth and biosynthesis in yeasts is in its early stages but holds the potential to be transformative in the coming decade. This review discusses the advances, challenges, and future perspectives in methanol-based biomanufacturing and innovative initiatives in formatotrophy in yeasts. Although it is a long way off, developments in synthetic biology assisted evolutionary engineering and artificial pathways will fill up the gaps in the scalability of C1-based bioprocesses, transforming yeasts into a reliable, climate-neutral, and resource-efficient platform for the green bioeconomy of the future.
酵母作为C1细胞工厂:将甲醇和甲酸酯转化为高价值化合物
微生物将二氧化碳和甲烷等温室气体转化为有价值的生化物质,似乎是制造业可持续脱碳的关键战略。许多尚未解决的技术限制仍然阻碍了这些单碳(C1)气基生物工艺的工业应用。将这些气体转化为液态C1化合物,如甲醇和甲酸盐,有助于减少排放,关闭碳循环。某些工业酵母具有耐受和同化甲醇和甲酸的内在能力,这为生态高效地利用这些化合物开辟了一条有吸引力的途径。为了提高酵母以c1为基础的生物制造潜力,合成甲基化已在多功能非甲基化基础上发展起来。策略性的非理性基因组工程和菌株进化结合合理的设计,揭示了隐藏的c1途径和底物耐受性机制。以甲醇为基础的生物生产的发展包括简单有机酸,具有工业规模扩大的明确前景,以及具有复杂途径的复杂聚酮的概念验证研究。生物生产的最新进展表明,模块化共培养工程和生物合成途径与C1代谢的过氧化物酶体偶联等技术取得了令人鼓舞的成果。酵母中以甲酸盐为基础的生长和生物合成尚处于早期阶段,但在未来十年具有变革性的潜力。本文综述了以甲醇为基础的生物制造的进展、挑战和未来前景,以及酵母形成物的创新举措。虽然还有很长的路要走,但合成生物学辅助进化工程和人工途径的发展将填补基于c1的生物过程可扩展性的空白,将酵母转变为可靠的、气候中性的、资源高效的平台,为未来的绿色生物经济服务。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biotechnology advances
工程技术-生物工程与应用微生物
CiteScore
25.50
自引率
2.50%
发文量
167
审稿时长
37 days
期刊介绍:
Biotechnology Advances is a comprehensive review journal that covers all aspects of the multidisciplinary field of biotechnology. The journal focuses on biotechnology principles and their applications in various industries, agriculture, medicine, environmental concerns, and regulatory issues. It publishes authoritative articles that highlight current developments and future trends in the field of biotechnology. The journal invites submissions of manuscripts that are relevant and appropriate. It targets a wide audience, including scientists, engineers, students, instructors, researchers, practitioners, managers, governments, and other stakeholders in the field. Additionally, special issues are published based on selected presentations from recent relevant conferences in collaboration with the organizations hosting those conferences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


