A multi reaction kinetic model to describe the enzymatic transesterification reaction of jatropha oil using a fermented solid containing lipases
IF 7.5
3区 环境科学与生态学
Q2 ENERGY & FUELS
引用次数: 0
Abstract
The advancement of more precise tools for sustainable process design in enzymatic biodiesel synthesis from renewable sources is crucial. Kinetics of solvent-free transesterification reactions were conducted across a temperature spectrum from 30 °C to 60 °C, utilizing Jatropha curcas oil (TG) and ethanol as substrates, alongside a fermented solid by Rhizopus homothallicus as the biocatalyst. The dynamics of chemical species concentrations were monitored through High-performance Thin-Layer Chromatography. Maximum productivities were achieved at 35 °C and 60 °C for biodiesel (293.24 and 299.02 g kg biocat−1 h−1, respectively), at 40 °C for diglycerides (1018.36 g kg biocat−1 h−1), and at 35 °C for monoglycerides (560.75 g kg biocat−1 h−1). Maximum yields were determined at 30 °C for fatty acid ethyl esters (0.56 g gTG−1), and at 40 °C for diglycerides (0.53 g gTG−1) and monoglycerides (0.30 g gTG−1). Based on the experimental findings, a kinetic model was formulated encompassing three reversible transesterification reactions. Individual reactions were structured following classical biochemical kinetics, inclusive of ethanol inhibition. Model fitting was executed through non-linear multivariable regression techniques, with the minimum of the average coefficient of variation of the residuals (ACVR) serving as the objective function. The resulting fit of the kinetic model to the experimental data proved satisfactory, with an ACVR of less than 5 % across all instances. Notably, the maximum biodiesel productivity, obtained in this work, represented the highest value, compared to other related studies, using a fermented solid as a biocatalyst.
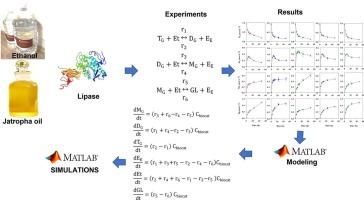
用含脂肪酶的发酵固体描述麻疯树油酶促酯交换反应的多反应动力学模型
更精确的工具的进步,可持续的过程设计酶合成生物柴油从可再生资源是至关重要的。在30 ~ 60℃的温度范围内,以麻疯树油(TG)和乙醇为底物,以同质根霉(Rhizopus homthallicus)发酵固体为生物催化剂,进行了无溶剂酯交换反应动力学研究。通过高效薄层色谱法监测化学物质浓度的动态变化。生物柴油在35°C和60°C的条件下(分别为293.24和299.02 g kg biocat - 1 h - 1),双甘油酯在40°C的条件下(1018.36 g kg biocat - 1 h - 1),单甘油酯在35°C的条件下(560.75 g kg biocat - 1 h - 1)达到最大生产率。在30°C时测定脂肪酸乙酯(0.56 g gTG−1)的最大产率,在40°C时测定二甘油酯(0.53 g gTG−1)和单甘油酯(0.30 g gTG−1)的最大产率。根据实验结果,建立了包含三个可逆酯交换反应的动力学模型。个体反应遵循经典生化动力学,包括乙醇抑制。采用非线性多变量回归技术进行模型拟合,以残差平均变异系数(ACVR)的最小值作为目标函数。动力学模型与实验数据的拟合结果令人满意,所有实例的ACVR均小于5%。值得注意的是,与使用发酵固体作为生物催化剂的其他相关研究相比,本研究中获得的最大生物柴油产量代表了最高的价值。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Carbon Resources Conversion
Materials Science-Materials Science (miscellaneous)
CiteScore
9.90
自引率
11.70%
发文量
36
审稿时长
10 weeks
期刊介绍:
Carbon Resources Conversion (CRC) publishes fundamental studies and industrial developments regarding relevant technologies aiming for the clean, efficient, value-added, and low-carbon utilization of carbon-containing resources as fuel for energy and as feedstock for materials or chemicals from, for example, fossil fuels, biomass, syngas, CO2, hydrocarbons, and organic wastes via physical, thermal, chemical, biological, and other technical methods. CRC also publishes scientific and engineering studies on resource characterization and pretreatment, carbon material innovation and production, clean technologies related to carbon resource conversion and utilization, and various process-supporting technologies, including on-line or off-line measurement and monitoring, modeling, simulations focused on safe and efficient process operation and control, and process and equipment optimization.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


