Zwitterionic resorcinarene bifunctional organocatalyst for the cycloaddition of carbon dioxide to epoxide
IF 5.8
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The cycloaddition of carbon dioxide (CO2) to epoxide (CCE) reaction is a highly attractive sustainable process due to its high atom economy and the versatility applications of the resulting cyclic carbonates. Conventional catalysts for CCE reactions were indispensable of halide cocatalyst, however, halides posed risk of corrosion to process steel equipment and the residue halide was environmentally harmful. To obviate halide, a new halide-free resorcinarene-based zwitterion (RES-Z) catalyst was designed and synthesized as organocatalyst for CCE reactions. The RES-Z catalyst exhibited excellent performance at 120 °C and 0.5 mol% catalyst loading under 1.0 MPa CO2 pressure for 12 h, the commercially epoxide bisphenol A diglycidyl ether and 12 terminal epoxides were converted into the corresponding cyclic carbonates with 73–99 % conversion and 99 % selectivity. Bifunctional catalytic mechanism was proposed and validated by NMR titrations where the cationic moiety activated the epoxide and the anionic moiety activated the CO2. Moreover, the catalyst demonstrated exceptional recyclability, exhibiting no decline in catalytic activity over five consecutive reaction cycles. These findings not only substantiate the efficacy of resorcinarene-based organocatalysts in halide-free catalytic systems but also advance the sustainable utilization of CO2 in chemical synthesis.
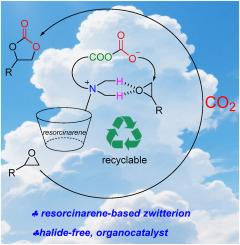
间苯二甲酸两性离子双功能有机催化剂,用于二氧化碳环加成环氧化物
二氧化碳(CO2)环加成到环氧化物(CCE)反应是一种极具吸引力的可持续过程,因为它具有高原子经济性和所产生的环碳酸盐的多用途应用。卤化物辅助催化剂是CCE反应中不可缺少的传统催化剂,但卤化物对炼钢设备有腐蚀风险,残留的卤化物对环境有害。为了消除卤化物,设计并合成了一种新型的无卤化物间苯二酚两性离子(RES-Z)催化剂,作为CCE反应的有机催化剂。RES-Z催化剂在120℃、0.5 mol%负载条件下表现优异,在1.0 MPa CO2压力下反应12 h,将工业环氧化合物双酚A二甘油酯和12种末端环氧化合物转化为相应的环碳酸盐,转化率为73 - 99%,选择性为99%。提出了双功能催化机理,并通过核磁共振滴定验证了其机理,其中阳离子部分活化环氧化物,阴离子部分活化CO2。此外,该催化剂表现出优异的可回收性,在连续五个反应周期中没有表现出催化活性的下降。这些发现不仅证实了间苯二酚类有机催化剂在无卤化物催化体系中的有效性,也为二氧化碳在化学合成中的可持续利用提供了新的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Sustainable Chemistry and Pharmacy
Environmental Science-Pollution
CiteScore
8.20
自引率
6.70%
发文量
274
审稿时长
37 days
期刊介绍:
Sustainable Chemistry and Pharmacy publishes research that is related to chemistry, pharmacy and sustainability science in a forward oriented manner. It provides a unique forum for the publication of innovative research on the intersection and overlap of chemistry and pharmacy on the one hand and sustainability on the other hand. This includes contributions related to increasing sustainability of chemistry and pharmaceutical science and industries itself as well as their products in relation to the contribution of these to sustainability itself. As an interdisciplinary and transdisciplinary journal it addresses all sustainability related issues along the life cycle of chemical and pharmaceutical products form resource related topics until the end of life of products. This includes not only natural science based approaches and issues but also from humanities, social science and economics as far as they are dealing with sustainability related to chemistry and pharmacy. Sustainable Chemistry and Pharmacy aims at bridging between disciplines as well as developing and developed countries.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


