Sugar nanocluster adhesive boosts wound healing in diabetic mice
IF 6.5
Q1 CHEMISTRY, APPLIED
Carbohydrate Polymer Technologies and Applications
Pub Date : 2025-07-08
DOI:10.1016/j.carpta.2025.100933
引用次数: 0
Abstract
In this study, we developed levan-catechol iron oxide (LC-IO) nanoclusters as multifunctional bioadhesive agents with strong tissue adhesion, antibacterial activity, and regenerative potential. The nanoclusters were fabricated via electrospray, yielding uniform, stable particles with catechol-mediated surface functionalization. Antibacterial evaluation using Escherichia coli (BL21) transformed with pTH-GFPuv showed a prolonged bacterial lag phase and reduced growth, indicating effective microbial suppression.
LC-IO nanoclusters demonstrated strong adhesive performance even at low concentrations (5 mg/mL), with lap shear stress and adhesion energy values of 30.13 kPa and 0.74 kJ/ m3, respectively more than double those of commercial fibrin glue (13.47 kPa and 0.33 kJ/m3).
Transcriptomic analysis of treated human dermal fibroblasts showed upregulation of genes related to extracellular matrix remodeling, angiogenesis, and cell migration. In vivo wound healing studies demonstrated that after 7 days, LC-IO–treated wounds achieved 86 % closure compared to 60 % in untreated controls. In a diabetic mouse model, 92 % of the wound area was healed by day 14 versus 62 % in untreated wounds.
These findings support LC-IO nanoclusters as a promising platform for wound healing applications, integrating strong adhesion, antimicrobial efficacy, and regenerative stimulation. Their ability to accelerate healing, particularly in diabetic wounds, highlights their potential for advanced wound care.
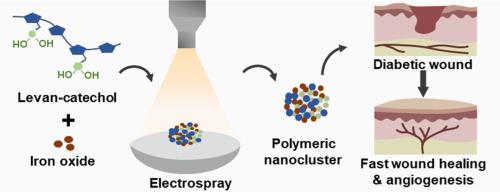
糖纳米团簇粘合剂促进糖尿病小鼠伤口愈合
在这项研究中,我们开发了左苯二酚氧化铁(LC-IO)纳米团簇作为多功能生物粘合剂,具有很强的组织粘附性、抗菌活性和再生潜力。通过电喷雾制备纳米团簇,得到均匀、稳定的颗粒,具有儿茶酚介导的表面功能化。用pTH-GFPuv转化大肠杆菌(BL21)进行抑菌评价,结果显示细菌滞后期延长,生长减慢,抑菌效果明显。LC-IO纳米团簇即使在低浓度(5 mg/mL)下也表现出较强的粘接性能,其粘接剪应力和粘接能分别为30.13 kPa和0.74 kJ/m3,是商用纤维蛋白胶(13.47 kPa和0.33 kJ/m3)的两倍多。经处理的人真皮成纤维细胞转录组学分析显示,与细胞外基质重塑、血管生成和细胞迁移相关的基因上调。体内伤口愈合研究表明,7天后,lc - io处理的伤口愈合率为86%,而未处理的对照组为60%。在糖尿病小鼠模型中,92%的伤口面积在第14天愈合,而未经治疗的伤口面积为62%。这些发现支持LC-IO纳米团簇作为伤口愈合应用的有前途的平台,集强粘附、抗菌功效和再生刺激于一体。它们加速愈合的能力,特别是在糖尿病伤口中,突出了它们在高级伤口护理方面的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


