All-optical mapping reveals distributed suppression of cortical sensory responses after optogenetic silencing
IF 8.4
1区 医学
Q1 CLINICAL NEUROLOGY
引用次数: 0
Abstract
Significance
We designed a novel all-optical tool to simultaneously silence neuronal activity at arbitrary sites on the dorsal cortex, and monitor the consequences of the manipulation. Optogenetic inhibition of primary sensory regions determined short and long-term dampening of the sensory response across a distributed cortical network.
Introduction
Many fundamental processes of brain computation, such as sensory perception and motor control, heavily rely on the mesoscopic dynamics of activity across the cerebral cortex. Manipulating mesoscale activity and observing its effects across multiple brain regions is crucial for understanding the causal link between cortical dynamics and behavior.
Objective
The goal of this study was to develop a novel all-optical system that allows inhibition of excitatory neurons while simultaneously monitoring cortical responses at arbitrary sites across the entire dorsal cortex of mice.
Methods
We combined wide-field imaging and optogenetics to create a mesoscale all-optical approach, enabling simultaneous monitoring and manipulation of cortical activity using light. Intravenous injection of two PHP.eB AAVs enabled the whole-brain co-expression of the red-shifted calcium indicator jRCaMP1b and the inhibitory actuator stGtACR2, with stable expression over several weeks. This system was calibrated, and the effects of inhibition on sensory responses were tested.
Results
Increasing laser power progressively reduced spontaneous activity at the site of irradiation. A single 5-s pulse on the barrel field cortex significantly decreased the amplitude of sensory-evoked responses, not only in the stimulated region but across the entire stimulated hemisphere.
Conclusions
This novel all-optical system enables targeted inhibition while concurrently monitoring mesoscale cortical activity. It provides insights into the dynamics of cortical circuits and offers a milestone for investigating the causal links between neuronal activity and behavior. Future research can use this tool to address sensory responsiveness impairments in neurological and neuropsychiatric disorders.
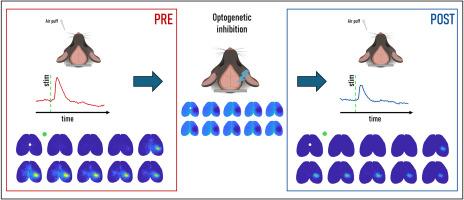
全光成像揭示光基因沉默后皮质感觉反应的分布抑制。
意义:我们设计了一种新型的全光学工具,可以同时使背皮层任意部位的神经元活动沉默,并监测操作的后果。主要感觉区域的光遗传抑制决定了通过分布式皮质网络的感觉反应的短期和长期抑制。大脑计算的许多基本过程,如感觉知觉和运动控制,严重依赖于大脑皮层活动的中观动力学。操纵中尺度活动并观察其在多个大脑区域的影响对于理解皮层动力学和行为之间的因果关系至关重要。目的:本研究的目的是开发一种新的全光学系统,该系统可以抑制兴奋性神经元,同时监测小鼠整个背皮层任意部位的皮层反应。方法:我们将宽视场成像和光遗传学相结合,创建了一种中尺度全光学方法,可以利用光同时监测和操纵皮层活动。静脉注射两支PHP。eB aav使红移钙指示物jRCaMP1b和抑制致动器stGtACR2在全脑共表达,并在数周内稳定表达。对该系统进行了标定,并测试了抑制对感觉反应的影响。结果:增加激光功率逐渐降低照射部位的自发活性。在桶状野皮层上施加一个5秒的脉冲,不仅在受刺激的区域,而且在整个受刺激的半球,都显著降低了感觉诱发反应的幅度。结论:这种新型的全光学系统可以在监测中尺度皮层活动的同时实现靶向抑制。它提供了对皮层回路动力学的见解,并为研究神经元活动和行为之间的因果关系提供了一个里程碑。未来的研究可以使用这个工具来解决神经和神经精神疾病的感觉反应障碍。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
Brain Stimulation
医学-临床神经学
CiteScore
13.10
自引率
9.10%
发文量
256
审稿时长
72 days
期刊介绍:
Brain Stimulation publishes on the entire field of brain stimulation, including noninvasive and invasive techniques and technologies that alter brain function through the use of electrical, magnetic, radiowave, or focally targeted pharmacologic stimulation.
Brain Stimulation aims to be the premier journal for publication of original research in the field of neuromodulation. The journal includes: a) Original articles; b) Short Communications; c) Invited and original reviews; d) Technology and methodological perspectives (reviews of new devices, description of new methods, etc.); and e) Letters to the Editor. Special issues of the journal will be considered based on scientific merit.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


