Combination targeted therapy with two biologic/targeted synthetic DMARDs in 1200 patients with immune mediated inflammatory diseases. A systematic literature review for current landscape in safety and efficacy
IF 8.3
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
Background
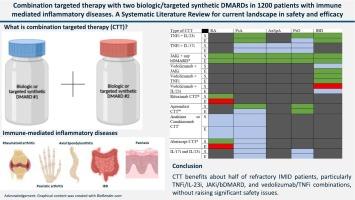
Immune-mediated inflammatory diseases (IMID) include rheumatoid arthritis (RA), psoriatic arthritis (PsA), axial spondyloarthritis (axSpA), Crohn's disease (CD), ulcerative colitis (UC), and psoriasis (PsO). While biologic (b) and targeted synthetic (ts) DMARDs are effective, nearly 60 % of patients fail to achieve low disease activity status. Combination targeted therapy (CTT) using concomitantly two different b- or ts-DMARDs has been explored, but results on safety and efficacy are unclear.
Objective
To systematically review the literature on CTT in IMID.
Methods
Following the PICO framework, we included literature of adult patients (≥18 years) with IMID receiving CTT. Three databases (PubMed, Scopus, Epistemonikos) were searched up to June 2024. Studies in non-English, pediatric populations, and non-approved treatments were excluded. Risk of bias was assessed using approved tools.
Results
Of 2038 records, 70 studies (6 RCTs, 11 cohorts, 22 case series, 31 case reports) involving 1200 patients were analyzed. About 75 % of them demonstrated low risk of bias. The most studied combinations were TNFi+IL/23i, JAKi+bDMARDs, and vedolizumab+TNFi. Approximately 40-60 % of patients with PsA, axSpA, and IBD with refractory disease improved with TNFi+IL/23i CTT. About half of patients with inflammatory arthritis and up to 80 % of IBD cases benefited with JAKi+bDMARD CTT, whereas favorable outcomes were observed in 30-50 % of IBD patients following Vedolizumab+TNFi CTT. Safety profiles were generally acceptable, without emerging signals so far.
Conclusion
CTT benefits about half of refractory IMID patients, particularly TNFi/IL-23i, JAKi/bDMARD, and vedolizumab/TNFi combinations, without raising significant safety issues. Further research is needed to clarify safety and efficacy across diseases.
两种生物/靶向合成dmard联合靶向治疗1200例免疫介导炎性疾病患者对安全性和有效性的现状进行系统的文献综述。
背景:免疫介导的炎症性疾病(IMID)包括类风湿关节炎(RA)、银屑病关节炎(PsA)、轴性脊柱炎(axSpA)、克罗恩病(CD)、溃疡性结肠炎(UC)和牛皮癣(PsO)。虽然生物制剂(b)和靶向合成(ts) dmard是有效的,但近60% %的患者未能达到低疾病活动状态。联合靶向治疗(CTT)联合使用两种不同的b-或ts- dmard已被探索,但安全性和有效性的结果尚不清楚。目的:系统回顾IMID中CTT的相关文献。方法:根据PICO框架,我们纳入了接受CTT治疗的IMID成年患者(≥18 岁)的文献。检索三个数据库(PubMed, Scopus, Epistemonikos)至2024年6月。非英语、儿科人群和未批准治疗的研究被排除在外。使用经批准的工具评估偏倚风险。结果:在2038份记录中,分析了涉及1200名患者的70项研究(6项随机对照试验,11个队列,22个病例系列,31个病例报告)。其中约75% %表现出低偏倚风险。研究最多的组合是TNFi+IL/23i, JAKi+bDMARDs和vedolizumab+TNFi。大约40- 60% %伴有难治性疾病的PsA、axSpA和IBD患者通过TNFi+IL/23i CTT得到改善。大约一半的炎症性关节炎患者和高达80% %的IBD病例受益于JAKi+bDMARD CTT,而30- 50% %的IBD患者在Vedolizumab+TNFi CTT中观察到良好的结果。安全概况总体上可以接受,到目前为止没有出现信号。结论:CTT使大约一半的难治性IMID患者受益,特别是TNFi/IL-23i, JAKi/bDMARD和vedolizumab/TNFi联合,没有引起明显的安全性问题。需要进一步的研究来澄清跨疾病的安全性和有效性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Autoimmunity reviews
医学-免疫学
CiteScore
24.70
自引率
4.40%
发文量
164
审稿时长
21 days
期刊介绍:
Autoimmunity Reviews is a publication that features up-to-date, structured reviews on various topics in the field of autoimmunity. These reviews are written by renowned experts and include demonstrative illustrations and tables. Each article will have a clear "take-home" message for readers.
The selection of articles is primarily done by the Editors-in-Chief, based on recommendations from the international Editorial Board. The topics covered in the articles span all areas of autoimmunology, aiming to bridge the gap between basic and clinical sciences.
In terms of content, the contributions in basic sciences delve into the pathophysiology and mechanisms of autoimmune disorders, as well as genomics and proteomics. On the other hand, clinical contributions focus on diseases related to autoimmunity, novel therapies, and clinical associations.
Autoimmunity Reviews is internationally recognized, and its articles are indexed and abstracted in prestigious databases such as PubMed/Medline, Science Citation Index Expanded, Biosciences Information Services, and Chemical Abstracts.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


