Leucine-Rich Repeat Containing 15 Promotes the Inflammatory Response in Rheumatoid Arthritis by Regulating NF-κB Pathway
Abstract
Purpose
To explore the influence and molecular mechanism of leucine-rich repeat containing 15 (LRRC15) in rheumatoid arthritis (RA) model induced by collagen-induced arthritis (CIA) in rats and interleukin-1 beta (IL-1β) treated fibroblast-like synoviocytes (FLSs).
Methods
LRRC15 expression was analyzed using reverse transcription quantitative polymerase chain reaction (RT-qPCR), western blot analysis, and immunohistochemistry. Hematoxylin-eosin (H&E) and safranin-O-green staining were performed to assess the pathological changes in the joint tissues of rats. The messenger ribonucleic acid (mRNA) and protein expression of interferon gamma (IFN-γ), interleukin (IL)-6, IL-1β, and IL-10 was detected by RT-qPCR, enzyme-linked immunosorbent assay (ELISA), and western blot. Cell proliferation and migration was surveyed using cell counting kit-8 (CCK-8), 5-Ethynyl-2′-deoxyuridine (EdU), and Transwell assays. Western blot was applied to detect the protein changes of nuclear factor kappaB (NF-κB) subunit p65, phosphorylated (p)-p65, inhibitor of kappa Bα (IκBα), phosphorylated inhibitor kappa Bα (p-IκBα), and nuclear factor erythroid 2-related factor 2 (Nrf2).
Results
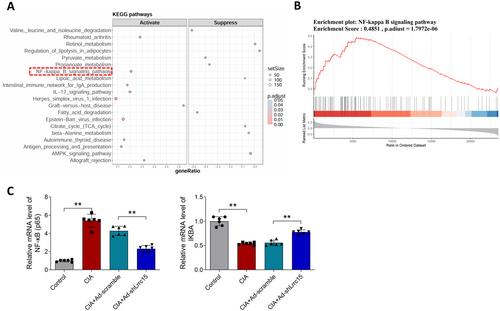
In CIA rats and IL-1β-treated FLSs, LRRC15 expression was upregulated. In vivo, Lrrc15 silencing prevented joint damage, inhibited the expression of IFN-γ, IL-6, IL-1β, and p65, and increased the expression of IL-10 and IκBα. In vitro, Lrrc15 silencing inhibited the proliferation, migration, and the expression of IFN-γ, IL-6, IL-1β, p-p65, and p-IκBα, and increased the expression of IL-10, IκBα, and Nrf2 in FLSs.
Conclusion
Lrrc15 silencing relieved joint damage and inflammatory response in RA, and this may be associated with the inhibition of the NF-κB pathway.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


