De novo drug design and biological evaluation of coumarin–pyrimidine co-drug derivatives as diabetic inhibitors: expanding a multi-algorithm approach with the integration of machine learning in pharmaceutical research†
IF 4.6
3区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
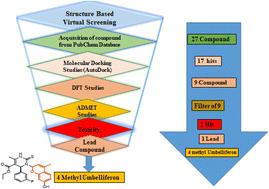
Optimization of hit compounds was carried out using the density functional theory. We introduce the coumarin-based co-drug by linkages (amide and oxime) of coumarins with pyrimidines to improve the pharmacokinetic activity of coumarin owing to the synergic effect of both collectively. Pharmacokinetic studies, including drug likeness, were performed using SWISSADMET, and toxicity was identified using a web-based server. Screened lead molecule W6 (C) was synthesized and characterized by NMR spectroscopy, and its anti-diabetic activity was evaluated by in vitro α-amylase inhibition. This study identified W6 as an active, new and more effective candidate for diabetes in drug discovery.
香豆素-嘧啶共药物衍生物作为糖尿病抑制剂的新药物设计和生物学评价:在药物研究中集成机器学习扩展多算法方法
利用密度泛函理论对hit化合物进行了优化。我们通过香豆素与嘧啶的键合(酰胺和肟),引入以香豆素为基础的联合药物,通过两者共同的协同作用来提高香豆素的药动学活性。使用SWISSADMET进行药代动力学研究,包括药物相似性,并使用基于web的服务器确定毒性。合成筛选到的铅分子W6 (C),通过核磁共振光谱对其进行表征,并通过体外α-淀粉酶抑制评价其抗糖尿病活性。本研究发现W6是一种活性的、新的、更有效的糖尿病药物候选物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

RSC Advances
chemical sciences-
CiteScore
7.50
自引率
2.60%
发文量
3116
审稿时长
1.6 months
期刊介绍:
An international, peer-reviewed journal covering all of the chemical sciences, including multidisciplinary and emerging areas. RSC Advances is a gold open access journal allowing researchers free access to research articles, and offering an affordable open access publishing option for authors around the world.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


