Pretreat immunosuppressants in whole blood without vortexing and centrifugation
IF 6.5
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
Background
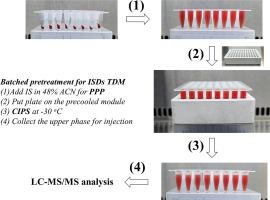
Precise measurement of immunosuppressant levels in whole blood is critical for monitoring post-transplant patient outcomes. Conventional protein precipitation (PP) methods, which rely on vortex mixing and centrifugation, present substantial limitations in terms of automation and scalability. To address these challenges, we developed a novel pretreatment strategy termed “Pseudo-Protein-Precipitation combined with Cold-Induced Phase Separation” (PPP+CIPS), designed to simplify sample processing and enhance high-throughput efficiency.
Results
The PPP+CIPS method employs 48 % acetonitrile to generate a semi-homogeneous blood suspension, enabling in-situ drug extraction via CIPS. Notably, this approach eliminates the need for vortexing and centrifugation—key bottlenecks in traditional therapeutic drug monitoring workflows. By leveraging 96-well plates and multi-channel pipettes, the protocol reduces pretreatment time to approximately one-third of that required by PP. Clinical validation (n = 288 in total) revealed strong concordance with established methods, with 94 % of tacrolimus, 95 % of cyclosporin A, and 92 % of sirolimus measurements falling within ±20 % agreement limits.
Significance
The PPP+CIPS strategy marks a significant leap forward in high-throughput therapeutic drug monitoring for immunosuppressants. Its seamless integration with 96-well formats and static processing workflows makes it a promising cornerstone for future automated and integrated TDM systems.
在全血中预处理免疫抑制剂,不进行旋流和离心
背景:精确测量全血免疫抑制剂水平对于监测移植后患者的预后至关重要。传统的蛋白质沉淀(PP)方法依赖于涡旋混合和离心,在自动化和可扩展性方面存在很大的局限性。为了应对这些挑战,我们开发了一种新的预处理策略,称为“伪蛋白质沉淀结合冷诱导相分离”(PPP+CIPS),旨在简化样品处理并提高高通量效率。结果PPP+CIPS方法采用48%乙腈制备半均质血悬液,可通过CIPS进行原位药物提取。值得注意的是,这种方法消除了传统治疗药物监测工作流程中的关键瓶颈——涡流和离心的需要。通过利用96孔板和多通道移液器,该方案将预处理时间减少到PP所需时间的约三分之一。临床验证(总共288例)显示与既定方法具有很强的一致性,94%的他克莫司,95%的环孢素A和92%的西罗莫司测量值在±20%的一致性范围内。PPP+CIPS策略标志着免疫抑制剂高通量治疗药物监测的重大飞跃。它与96井格式和静态处理工作流程的无缝集成使其成为未来自动化和集成TDM系统的基石。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


