Enzymatic DNA orthogonal chemistry for multi-cancer diagnosis
IF 19.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
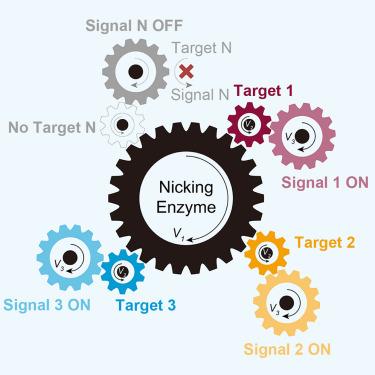
Orthogonal detection of multiple targets is essential for precision medicine, but achieving this in homogeneous systems is challenging owing to coordination issues regarding reagents and detection platforms. Here, we developed an enzymatic DNA orthogonal chemistry-based 3D spectral fingerprint (EzDo-CRAFT) platform, which integrates nicking endonuclease-based orthogonality with excitation-emission matrix (EEM) spectroscopy-based 3D signal resolution. Notably, we discovered that the nicking endonuclease Nt.BstNBI exhibits sensitivity to variable recognition sites and that their reaction processes are highly orthogonal, which laid the foundation for EzDo-CRAFT. Moreover, EEM spectroscopy effectively overcomes the limitations of spectral overlap and provides 3D spectral fingerprint features with high information density. The EzDo-CRAFT platform enabled one-pot detection of 10 targets across bladder cancer, prostate cancer, kidney cancer, and healthy individuals, using clinical urine samples, demonstrating high sensitivity (82.7%, 95% CI: 63.5%–93.5%) and specificity (81.8%, 95% CI: 47.8%–96.8%). This EzDo-CRAFT platform demonstrates an innovative application of nicking endonucleases and their potential for advancing precision medicine.
酶DNA正交化学用于多种肿瘤诊断
多靶点的正交检测对于精准医疗至关重要,但由于试剂和检测平台的协调问题,在同质系统中实现这一目标具有挑战性。在这里,我们开发了一个基于酶DNA正交化学的3D光谱指纹(EzDo-CRAFT)平台,该平台将基于切口内切酶的正交性与基于激发发射矩阵(EEM)光谱的3D信号分辨率相结合。值得注意的是,我们发现切口内切酶Nt.BstNBI对可变识别位点具有敏感性,并且它们的反应过程高度正交,这为EzDo-CRAFT奠定了基础。此外,EEM光谱有效克服了光谱重叠的限制,提供了信息密度高的三维光谱指纹特征。EzDo-CRAFT平台使用临床尿液样本,可一锅检测膀胱癌、前列腺癌、肾癌和健康个体的10个靶点,具有高灵敏度(82.7%,95% CI: 63.5%-93.5%)和特异性(81.8%,95% CI: 47.8%-96.8%)。这个EzDo-CRAFT平台展示了切口内切酶的创新应用及其推进精准医学的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


