One-shot synthesis of BN-embedded hexabenzocoronene via a dearomative triple borylation
IF 19.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The missing BN isostere of coronene, 2a1,6a1,10b1-triaza-2b,6b,10b-triborahexabenzo[a,d,g,j,m,p]coronene, was synthesized in a one-shot procedure from 2,4,6-tribenzhydryl-1,3,5-triazine via a dearomative triple borylation. In the crystalline state, the resulting nearly D3-symmetric molecule forms an efficient 3D π-stacking network, with disorder observed in the B–N and C=C bonds. Density functional theory (DFT) calculations of electronic couplings predict a promising potential for applications in organic semiconducting materials. The introduction of six additional methyl groups suppressed this disorder, enabling precise bond-length alternation analysis. Notably, the incorporation of three BN units into the spoke positions of the coronene core induced a paratropic ring current in the N3C3 central ring, a hypsochromic shift in absorption and emission maxima, and an enhanced photoluminescence quantum yield. Furthermore, selective single and double borylations of 2,4,6-tribenzhydryl-1,3,5-triazine were demonstrated, alongside a dearomative triple borylation of its benzothiophene analog. These findings may accelerate advances in both fundamental and applied chemistry based on BN/CC isosterism.
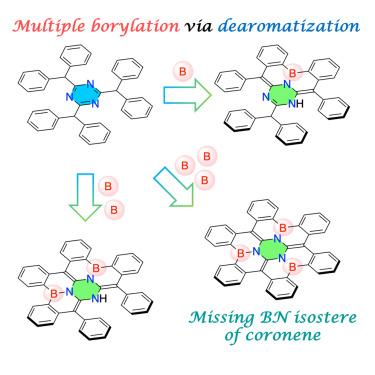
脱芳三硼化反应一次性合成bn包埋六苯并二苯甲醚
以2,4,6-三苯并基-1,3,5-三嗪为原料,经脱芳三硼化反应,一次合成了冠烯缺失的BN同分异构体2a1,6a1,10b1-三氮杂-2b,6b,10b-三硼己苯并[a,d,g,j,m,p]。在结晶状态下,得到的近乎三维对称的分子形成了一个高效的三维π堆叠网络,在B-N和C=C键中观察到无序。密度泛函理论(DFT)预测了电子耦合在有机半导体材料中的应用前景。另外六个甲基的引入抑制了这种紊乱,使精确的键长交替分析成为可能。值得注意的是,在日冕核心的辐条位置加入3个BN单元,在N3C3中心环上产生了顺向环电流,吸收和发射最大值发生了次色移,光致发光量子产率提高。此外,2,4,6-三苄基-1,3,5-三嗪的选择性单硼化和双硼化,以及苯并噻吩类似物的脱芳三硼化。这些发现可能会加速基于BN/CC同构关系的基础化学和应用化学的进展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


