Curcumin as a Cinnamoyl Transfer Reagent via C–C(CO) Bond Scissoring in the Microwave-Assisted Reaction with Hydroxy-p-QMs
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A microwave-assisted cascade conjugate addition–intramolecular cinnamoyl transfer (“cut and sew”) strategy involving 2-hydroxyphenyl-para-quinone methides (HPQMs) and curcumins, yielding cinnamoyl cinnamates bearing a diarylalkyl moiety in excellent yields and with broad substrate versatility, is reported. Mechanistic studies revealed a cascade Michael–hemiketalization–retro-Claisen reaction resulting in the C–C(CO) bond cleavage of curcumin. Furthermore, the cinnamoyl transfer products are amenable for a variety of synthetic transformations, highlighting their significance and synthetic utility.
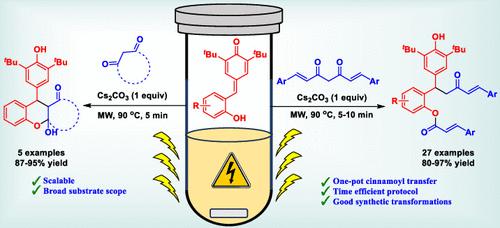
姜黄素与羟基-p- qms微波辅助反应中C-C(CO)键剪切作为肉桂基转移试剂。
本文报道了一种微波辅助级联共轭加成-分子内肉桂基转移(“切割和缝合”)策略,涉及2-羟基苯基对醌(HPQMs)和姜黄素,以优异的收率和广泛的底物多用途性生产具有二芳基烷基部分的肉桂基肉桂酸盐。机理研究揭示了一个级联的michael - hemiktalization - retroclisen反应导致姜黄素C-C(CO)键断裂。此外,肉桂酰转移产物可进行多种合成转化,突出了它们的重要性和合成实用性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


