Asymmetric biomimetic aldol reaction of glycinate enables highly efficient synthesis of chiral β-hydroxy-α-amino acid derivatives
IF 44.6
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Efficient biomimetic reactions provide a powerful platform for producing bioactive chiral compounds. Chiral β-hydroxy-α-amino acids display exceptionally high bioactivity and are key functional components of many marketed drugs. However, the efficient synthesis of chiral β-hydroxy-α-amino acids remains a long-standing challenge for both chemical and biological syntheses. Chemical synthesis is problematic due to low step- and atom-efficiencies, high costs and environmental hazards. The enzymatic aldol reaction of glycine can make β-hydroxy-α-amino acids in one step, but it is constrained by issues such as limited substrate scope, unsatisfactory stereoselectivity and low conversion. Here we have successfully realized the biomimetic aldol reaction of glycinate with aldehydes, using 0.01–1.0 mol% of a chiral pyridoxal as the catalyst to produce a remarkably broad range of chiral β-hydroxy-α-amino esters. Moreover, a high-diversity parallel asymmetric synthesis has also been tested using over 1,100 aldehydes and it yielded over 1,700 chiral β-hydroxy-α-amino acid esters, demonstrating its important potential for drug innovation. Chemical synthesis of chiral β-hydroxy-α-amino acids usually requires multiple steps. Now a biomimetic enantioselective aldol reaction of glycinate and aldehydes catalysed by a chiral pyridoxal has been achieved, providing efficient access to a large number of chiral β-hydroxy-α-amino esters.
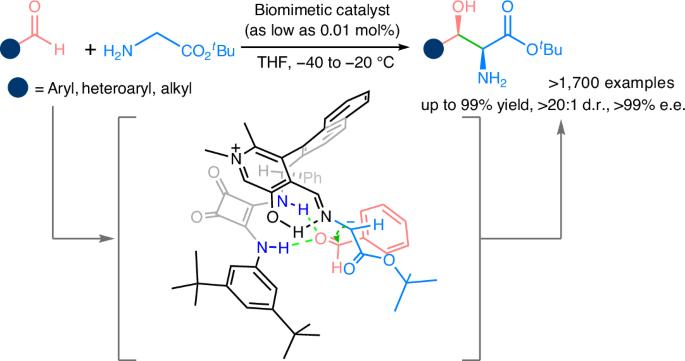

甘氨酸不对称仿生醛醇反应可以高效合成手性β-羟基-α-氨基酸衍生物
高效的仿生反应为制备具有生物活性的手性化合物提供了强有力的平台。手性β-羟基-α-氨基酸具有极高的生物活性,是许多上市药物的关键功能成分。然而,手性β-羟基-α-氨基酸的高效合成仍然是化学和生物合成领域的一个长期挑战。化学合成由于步骤和原子效率低、成本高和环境危害而存在问题。甘氨酸酶法醛醇反应可一步制得β-羟基-α-氨基酸,但存在底物范围有限、立体选择性不理想、转化率低等问题。本研究成功地实现了甘氨酸与醛类的仿生醛醇反应,以0.01 ~ 1.0 mol%的手性吡哆醛为催化剂,生成了范围非常广泛的手性β-羟基-α-氨基酯。此外,高多样性平行不对称合成也被测试使用超过1100个醛,产生超过1700个手性β-羟基-α-氨基酸酯,显示其在药物创新方面的重要潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


