NLRP3 inflammasome–driven hemophagocytic lymphohistiocytosis occurs independent of IL-1β and IL-18 and is targetable by BET inhibitors
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
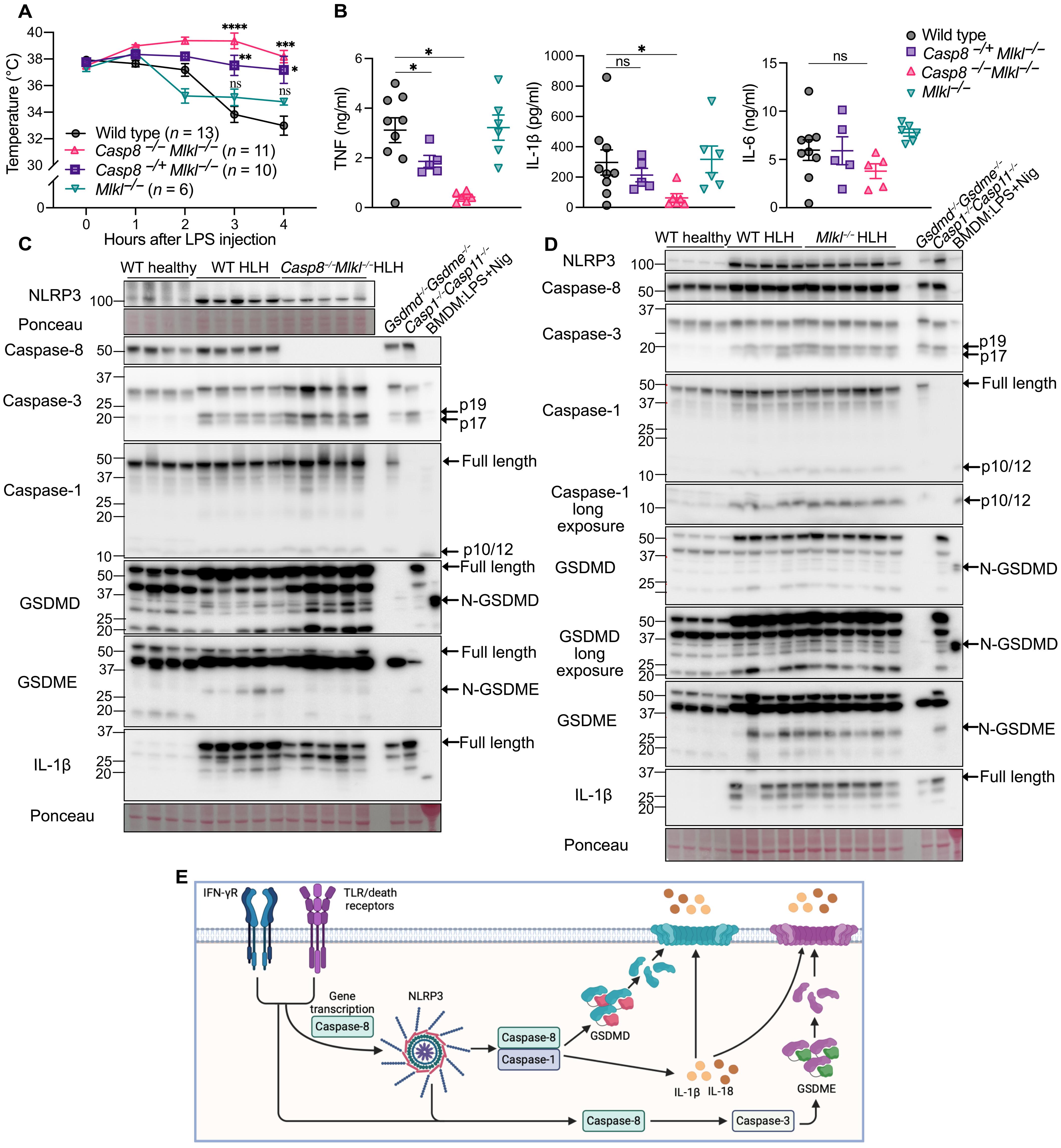
Hemophagocytic lymphohistiocytosis (HLH) is a potentially fatal cytokine storm syndrome. Its high mortality rate reflects limited therapeutic options and a poor understanding of disease-causing signaling. We show that the NLRP3 inflammasome is responsible for increased mortality in a model of secondary HLH (sHLH). Unexpectedly, neither deletion of the NLRP3-activated pyroptotic effector GSDMD nor combined deletion of the inflammasome-activated cytokines interleukin-1β (IL-1β) and IL-18 conferred strong protection from sHLH. Instead, co-deletion of GSDMD and caspase-8–activated GSDME limited sHLH-driven lethality, demonstrating redundancy in the pyroptotic machinery required to induce sHLH. We also found that bromodomain and extraterminal domain (BET) inhibitors prevent NLRP3-driven pyroptosis, which acted by blocking inflammasome priming. BET inhibitors prevented increased NLRP3 levels in diseased tissue, limited the production of sHLH-associated IL-1β, interferon-γ, and tumor necrosis factor, and protected from sHLH pathogenesis. These findings suggest that targeting NLRP3 could limit sHLH and identify clinically relevant bromodomain-selective BET inhibitors capable of eliminating NLRP3-driven pyroptosis and the sHLH cytokine storm.
NLRP3炎性小体驱动的噬血细胞淋巴组织细胞作用不依赖于IL-1β和IL-18,并可被BET抑制剂靶向
噬血细胞淋巴组织细胞增多症(HLH)是一种潜在致命的细胞因子风暴综合征。它的高死亡率反映了有限的治疗选择和对致病信号的了解不足。我们发现NLRP3炎性体是继发性HLH (sHLH)模型中死亡率增加的原因。出乎意料的是,无论是nlrp3激活的热亡效应物GSDMD的缺失,还是炎性小体激活的细胞因子白素-1β (IL-1β)和IL-18的联合缺失,都没有对sHLH产生强大的保护作用。相反,GSDMD和caspase-8激活的GSDME的共缺失限制了sHLH驱动的致死性,证明了诱导sHLH所需的焦亡机制的冗余性。我们还发现溴域和外域(BET)抑制剂可以阻止nlrp3驱动的焦亡,这是通过阻断炎性体的启动来实现的。BET抑制剂阻止病变组织中NLRP3水平的升高,限制sHLH相关IL-1β、干扰素-γ和肿瘤坏死因子的产生,并保护sHLH的发病机制。这些发现表明,靶向NLRP3可以限制sHLH,并鉴定出能够消除NLRP3驱动的焦亡和sHLH细胞因子风暴的临床相关溴域选择性BET抑制剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


