Bromodomain-containing proteins interact with a non-canonical RNA polymerase II kinase to maintain gene expression upon heat stress
IF 13.6
1区 生物学
Q1 PLANT SCIENCES
引用次数: 0
Abstract
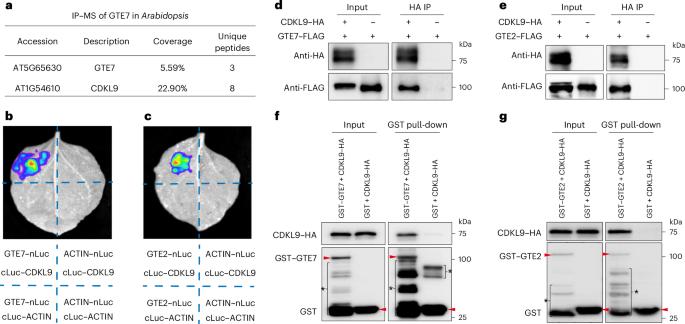
Phosphorylation at the carboxy-terminal domain of the largest subunit of RNA polymerase II plays a critical role in transcription, and histone acetylation is correlated with active transcription. However, the regulatory mechanisms by which histone acetylation modulates RNA polymerase II phosphorylation in plants remain unclear. Here we show that two functionally redundant bromodomain-containing proteins, global transcription factor group E2 (GTE2) and GTE7, can bind to acetylated histone H4. Both GTE2 and GTE7 interact with cyclin-dependent kinase-like 9 (CDKL9), which belongs to a plant-specific CDKL group. Unlike canonical CDKs, CDKL9 functions in a cyclin- and CDK-activating-kinase-independent manner and can phosphorylate at least the serine 2 and serine 5 residues of the carboxy-terminal domain in vitro. The GTE2/GTE7–CDKL9 complex is required to maintain serine 2 and serine 5 phosphorylation under heat stress. Consistently, loss-of-function gte2/gte7 and cdkl9 mutants show similar heat-sensitive phenotypes. We also demonstrate that the acetylated-histone-binding activity of GTE7 is essential for the association of CDKL9 with chromatin and for plant heat tolerance. Together, these findings provide mechanistic insight into transcriptional regulation via histone acetylation in response to heat stress and suggest that plants might have evolved a unique group of carboxy-terminal domain kinases for stress tolerance. Zheng et al. identify a non-canonical RNA polymerase II kinase, CDKL9, that tends to act when plants are under heat stress. Acetylated-histone-binding proteins GTE2 and GTE7 help CDKL9 localize to targets. All of these proteins are important for plant heat tolerance.

含溴结构域的蛋白与非规范RNA聚合酶II激酶相互作用,在热应激下维持基因表达
RNA聚合酶II最大亚基羧基末端的磷酸化在转录中起关键作用,组蛋白乙酰化与活性转录相关。然而,植物中组蛋白乙酰化调节RNA聚合酶II磷酸化的调控机制尚不清楚。在这里,我们发现两个功能冗余的含溴结构域的蛋白,全局转录因子组E2 (GTE2)和GTE7,可以结合到乙酰化组蛋白H4。GTE2和GTE7与细胞周期蛋白依赖性激酶样9 (CDKL9)相互作用,CDKL9属于植物特异性CDKL群。与典型CDKs不同,CDKL9以细胞周期蛋白和cdk激活激酶独立的方式起作用,并且在体外可以磷酸化至少羧基末端结构域的丝氨酸2和丝氨酸5残基。GTE2/ GTE7-CDKL9复合体是维持热应激下丝氨酸2和丝氨酸5磷酸化所必需的。功能缺失的gte2/gte7和cdkl9突变体始终表现出相似的热敏表型。我们还证明了GTE7的乙酰化组蛋白结合活性对于CDKL9与染色质的关联和植物的耐热性至关重要。总之,这些发现提供了通过组蛋白乙酰化来应对热胁迫的转录调控机制,并表明植物可能已经进化出一组独特的羧基末端结构域激酶来抵抗胁迫。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Plants
PLANT SCIENCES-
CiteScore
25.30
自引率
2.20%
发文量
196
期刊介绍:
Nature Plants is an online-only, monthly journal publishing the best research on plants — from their evolution, development, metabolism and environmental interactions to their societal significance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


