Interrupted Houben-Hoesch Nucleophilic Cascade Strategy for the Synthesis of 6,7-Oxoannulated Indoles
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
An interrupted Houben-Hoesch cascade strategy is reported for the synthesis of 6,7-oxoannulated indoles, analogs of 6,7-carboannulated indole natural products, from pyrrolyl succinonitrile silyl ethers. The cascade proceeds through an initial nucleophilic attack by the pyrrole on a Lewis acid-activated nitrilium, followed by a second nucleophilic attack on the resulting iminium intermediate by an in situ-deprotected alcohol. The strategy enables the synthesis of a broad range of substituted dihydrofurano- and tetrahydropyranoindoles, scaffolds that are synthetically challenging by other means, in a single step.
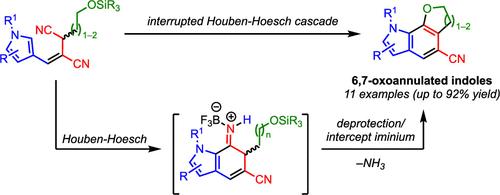
中断Houben-Hoesch亲核级联策略合成6,7-氧环吲哚。
报道了一种中断的Houben-Hoesch级联策略,用于从吡咯基琥珀腈硅基醚合成6,7-氧环吲哚,6,7-碳环吲哚天然产物的类似物。该级联反应首先由吡咯对Lewis酸活化的腈进行亲核攻击,然后由原位去保护的醇对生成的中间体进行第二次亲核攻击。该策略能够在一个步骤中合成范围广泛的取代二氢呋喃和四氢吡喃吲哚,这些支架是通过其他方法合成具有挑战性的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


