Current and future perspective of registry utilisation for regulatory settings in Japan: pharmaceuticals, medical devices, and regenerative medicine products.
IF 2.5
Q1 HEALTH POLICY & SERVICES
Journal of Pharmaceutical Policy and Practice
Pub Date : 2025-07-04
eCollection Date: 2025-01-01
DOI:10.1080/20523211.2025.2523935
引用次数: 0
Abstract
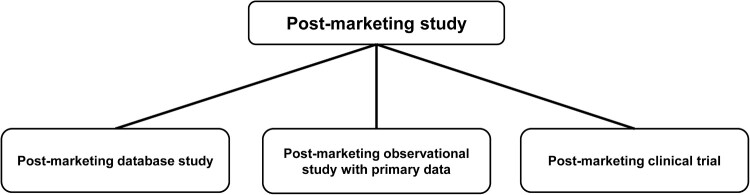
Considerable attention has been paid to the utilisation of real-world data throughout the lifecycle of pharmaceuticals, medical devices, and regenerative products. In Japan, regulatory initiatives have been implemented to promote the utilisation of real-world data and evidence for regulatory decision-making, especially focusing on the use of disease registries. In this brief article, we outline regulatory initiatives, summarise the current status of registry utilisation in Japanese regulatory settings, and offer points to consider and perspectives on registry utilisation for stakeholder collaboration.
当前和未来的前景注册利用的监管设置在日本:药品,医疗设备和再生医学产品。
在药品、医疗器械和再生产品的整个生命周期中,对真实世界数据的利用已经引起了相当大的关注。在日本,已经实施了监管举措,以促进利用真实世界的数据和证据进行监管决策,特别是侧重于疾病登记的使用。在这篇简短的文章中,我们概述了监管举措,总结了日本监管环境中注册管理机构利用的现状,并提供了关于注册管理机构利用利益相关者合作的考虑点和观点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Pharmaceutical Policy and Practice
Health Professions-Pharmacy
CiteScore
4.70
自引率
9.50%
发文量
81
审稿时长
14 weeks
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


