Enhancement of Ni oxidation tolerance in Ni/GDC cathode surface during CO2/H2O electrolysis in SOEC
IF 8.3
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The enhancement of Ni oxidation tolerance in a Ni/GDC cathode during CO2/H2O electrolysis in a solid oxide electrolysis cell was investigated through experiments and first-principle density functional theory (DFT) calculations. Ni oxidation was considerably suppressed in the Ni/GDC cathode in the absence of H2 during CO2/H2O electrolysis, whereas Ni oxidation occurred in the Ni/YSZ cathode. A decrease in the H2/CO ratio in Ni/GDC suggested that the reverse water–gas shift reaction was enhanced owing to the presence of active GDC surfaces with extra vacancies. DFT calculations revealed that the activation barriers for cathodic reactions at triple-phase boundary (TPB)—including CO2 adsorption on Ni, CO2 dissociation into CO and O, and O migration from the Ni surface to the electrolyte—were nearly identical in Ni/YSZ and Ni/GDC. Instead, O migration from the Ni surface to extra vacancies in the GDC surface improved Ni oxidation resistance in Ni/GDC during CO2/H2O electrolysis.
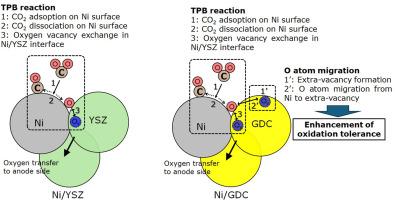
SOEC中CO2/H2O电解过程中Ni/GDC阴极表面Ni抗氧化能力的增强
通过实验和第一性原理密度泛函理论(DFT)计算,研究了固体氧化物电解池中CO2/H2O电解过程中Ni/GDC阴极中Ni耐氧化性的增强。在CO2/H2O电解过程中,在没有H2的情况下,Ni/GDC阴极的Ni氧化被明显抑制,而Ni/YSZ阴极则发生了Ni氧化。Ni/GDC中H2/CO比的降低表明,由于活性GDC表面存在额外的空位,从而增强了逆向水气转换反应。DFT计算表明,在Ni/YSZ和Ni/GDC中,三相边界(TPB)阴极反应的激活势垒(包括CO2在Ni上的吸附、CO2解离成CO和O以及O从Ni表面向电解质的迁移)几乎相同。相反,在CO2/H2O电解过程中,O从Ni表面迁移到GDC表面的额外空位,提高了Ni/GDC中Ni的抗氧化性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

International Journal of Hydrogen Energy
工程技术-环境科学
CiteScore
13.50
自引率
25.00%
发文量
3502
审稿时长
60 days
期刊介绍:
The objective of the International Journal of Hydrogen Energy is to facilitate the exchange of new ideas, technological advancements, and research findings in the field of Hydrogen Energy among scientists and engineers worldwide. This journal showcases original research, both analytical and experimental, covering various aspects of Hydrogen Energy. These include production, storage, transmission, utilization, enabling technologies, environmental impact, economic considerations, and global perspectives on hydrogen and its carriers such as NH3, CH4, alcohols, etc.
The utilization aspect encompasses various methods such as thermochemical (combustion), photochemical, electrochemical (fuel cells), and nuclear conversion of hydrogen, hydrogen isotopes, and hydrogen carriers into thermal, mechanical, and electrical energies. The applications of these energies can be found in transportation (including aerospace), industrial, commercial, and residential sectors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


