Stimuli-sensitive hyaluronic acid hydrogels for localized and controlled release of antibodies
IF 4.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2025-07-08
DOI:10.1016/j.ejpb.2025.114804
引用次数: 0
Abstract
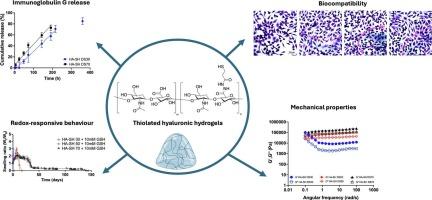
Stimuli-sensitive hydrogels are utilized in therapeutic applications for their ability to function as controlled drug delivery systems, particularly as delivery platforms for antibodies in cancer treatment. Their adaptive properties, including biocompatibility, high water retention, and tunable mechanical strength, make them well-suited for local and sustained drug release. In this study, redox-sensitive hydrogels based on thiolated hyaluronic acid (HA-SH) were synthetized as tunable platforms for controlled antibody delivery in cancer therapy. HA-SH hydrogels with different degrees of substitution (DS30, DS50 and DS70) exhibited distinct structural and mechanical properties, with HA-SH DS70 forming a denser network and demonstrating greater stability compared to HA-SH DS30 and DS50. Swelling and degradation studies confirmed redox responsiveness of the gels, with DS30 gel degrading faster than DS50 and DS70 gels in reductive environments. Rheological analysis further showed that higher cross-linking density in DS70 gels enhanced viscosity and mechanical strength compared to DS50 and DS30. Immunoglobulin G (IgG), used as a model drug for immunotherapeutic agents, was loaded into DS30 and DS70 hydrogels. The release followed zero-order kinetics at pH 7.4, highlighting the influence of the polysaccharide intrinsic anionic properties. DS30 hydrogels demonstrated sustained release (85 ± 6 % in 9 days), while DS70 exhibited faster release (71 ± 7 % in 5 days). The IgG release kinetics relied on a dual mechanism involving the combination of gel erosion (depending on DS and structural features), as well as IgG poly-charged nature and its ionic interactions with the hyaluronic acid polymeric network, as highlighted by rheological measurements and differential scanning calorimetry (DSC) analysis. Overall, the study highlights the potential of HA-SH hydrogels as customizable and localized immunotherapeutic delivery systems for controlled and precise cancer treatment.
刺激敏感的透明质酸水凝胶,用于局部和控制抗体的释放
刺激敏感水凝胶由于其作为受控药物输送系统的能力而被用于治疗应用,特别是作为癌症治疗中抗体的输送平台。它们的适应性,包括生物相容性、高保水性和可调节的机械强度,使它们非常适合局部和持续的药物释放。在这项研究中,基于硫代透明质酸(HA-SH)的氧化还原敏感水凝胶被合成为癌症治疗中可调节的抗体递送平台。不同取代度的HA-SH水凝胶(DS30、DS50和DS70)表现出不同的结构和力学性能,与HA-SH DS30和DS50相比,HA-SH DS70形成更密集的网络,表现出更大的稳定性。溶胀和降解研究证实了凝胶的氧化还原反应性,在还原环境中,DS30凝胶的降解速度快于DS50和DS70凝胶。流变学分析进一步表明,与DS50和DS30相比,DS70凝胶中较高的交联密度提高了粘度和机械强度。将免疫球蛋白G (IgG)作为免疫治疗剂的模型药物,装入DS30和DS70水凝胶中。pH为7.4时,其释放遵循零级动力学,突出了多糖固有阴离子性质的影响。DS30水凝胶缓释率为85±6% (9 d), DS70水凝胶缓释率为71±7% (5 d)。IgG释放动力学依赖于双重机制,包括凝胶侵蚀(取决于DS和结构特征),以及IgG的多电荷性质及其与透明质酸聚合物网络的离子相互作用,如流变学测量和差示扫描量热法(DSC)分析所强调的那样。总的来说,该研究强调了HA-SH水凝胶作为可定制和局部免疫治疗递送系统的潜力,可用于控制和精确的癌症治疗。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


