Giardia-induced Type 2 mucosal immunity attenuates intestinal inflammation caused by co-infection or colitis in mice
IF 19.4
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
Abstract
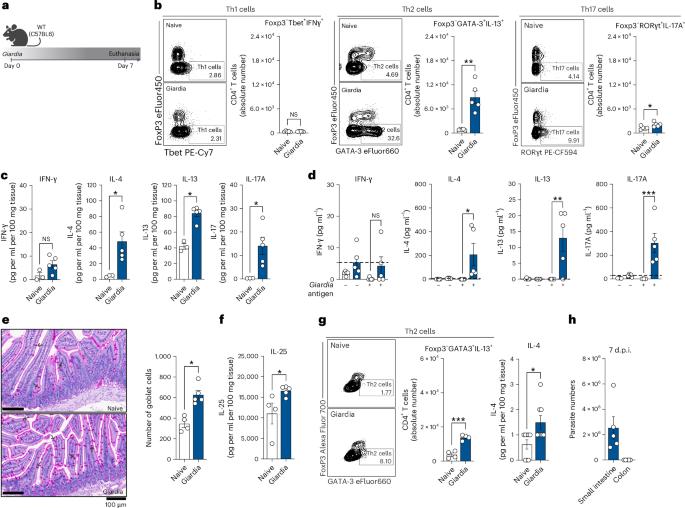
Diarrhoeal diseases are the second leading cause of death in children worldwide. Epidemiological studies show that co-infection with the protozoan parasite Giardia intestinalis decreases diarrhoeal severity. Here we show a high incidence of asymptomatic Giardia infection in school-aged children from Nigeria. In a mouse model, Giardia induced a Type 2 mucosal immune response, characterized by antigen-specific Th2 cells, IL-25, Type 2 cytokines, and goblet cell hyperplasia. Single-cell RNA sequencing and multiparameter flow cytometry revealed expansion of IL-10-producing Th2 cells, which promoted parasite persistence and protected against Toxoplasma gondii-induced ileitis and dextran sulfate sodium-induced colitis. This protective effect was STAT6 dependent, as IL-4R blockade or STAT6 deficiency impaired IL-10+ Th2 responses, resulting in Th1/Th17-driven tissue damage, inflammation and clearance of Giardia infection. Our findings demonstrate that Giardia reshapes mucosal immunity toward a Type 2 response, facilitating parasitism and conferring mutualistic protection from inflammatory pathologies, highlighting a key role for protists in mucosal defence regulation. A host–protist interaction regulates mucosal immunity to promote parasitism which also reduces the risk of inflammatory-driven pathologies of the gut.

贾第鞭毛虫诱导的2型粘膜免疫可减轻小鼠共感染或结肠炎引起的肠道炎症
腹泻病是全世界儿童死亡的第二大原因。流行病学研究表明,与原生动物寄生虫肠贾第虫合并感染可降低腹泻严重程度。在这里,我们显示了尼日利亚学龄儿童无症状贾第虫感染的高发。在小鼠模型中,贾第鞭毛虫诱导了2型粘膜免疫反应,其特征是抗原特异性Th2细胞、IL-25、2型细胞因子和杯状细胞增生。单细胞RNA测序和多参数流式细胞术显示,产生il -10的Th2细胞扩增,促进了寄生虫的持久性,并对刚地弓形虫诱导的回肠炎和葡聚糖硫酸钠诱导的结肠炎具有保护作用。这种保护作用依赖于STAT6,因为IL-4R阻断或STAT6缺乏会损害IL-10+ Th2反应,导致Th1/ th17驱动的组织损伤、炎症和贾第鞭毛虫感染的清除。我们的研究结果表明,贾第鞭毛虫重塑了黏膜对2型反应的免疫,促进了寄生,并赋予了对炎症病理的互惠保护,突出了原生生物在粘膜防御调节中的关键作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


