Development of multiplex recombinase polymerase amplification for the rapid detection of five carbapenemase (blaKPC, blaNDM, blaOXA-48-like, blaIMP, and blaVIM) and 10 mcr (mcr-1 to mcr-10) genes in blood cultures
IF 4.6
2区 医学
Q1 INFECTIOUS DISEASES
International Journal of Antimicrobial Agents
Pub Date : 2025-07-05
DOI:10.1016/j.ijantimicag.2025.107567
引用次数: 0
Abstract
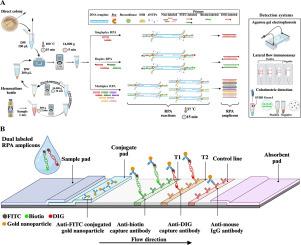
The emergence of plasmid-encoded carbapenemase and mobile colistin resistance (mcr) genes poses a significant challenge in controlling the spread of multidrug-resistant Gram-negative bacteria. Addressing this issue requires the development of rapid, accurate, and cost-effective tools for gene detection. For the first time, this study reports three multiplex recombinase polymerase amplification (RPA) assays, each designed to detect five resistance genes: carbapenemase (blaKPC, blaNDM, blaOXA-48-like, blaIMP, and blaVIM), mcr-1 to mcr-5, and mcr-6 to mcr-10. Using agarose gel electrophoresis, all 15 target genes were successfully amplified by the three assays, demonstrating the potential of these assays for integration with rapid reporting platforms. To increase their applicability, the assays were combined with SYBRⓇ Green I for visual identification of all 15 target genes and with lateral flow immunoassays (LFIAs) for detection of two carbapenemase (blaNDM and blaOXA-48-like) and two mcr genes (mcr-1 and mcr-3) genes. Specificity testing showed that RPA-SYBRⓇ Green I and RPA-LFIAs produced no cross-reactivity among the target genes. The limit of detection for RPA-SYBRⓇ Green I, for all genes, ranged from 2 × 100 to 2 × 102 CFU/reaction, and for RPA-LFIAs from 2 × 100 to 2 × 103 CFU/reaction. The developed RPA-SYBRⓇ Green I and RPA-LFIAs successfully detected 15 and four target genes, from positive haemoculture bottles. These assays offer a promising approach for point-of-care testing. Providing a valuable tool for antimicrobial resistance surveillance and timely guidance for effective antibiotic intervention.
建立用于快速检测血培养物中5种碳青霉烯酶(blaKPC、blaNDM、blaoxa -48样、blaIMP和blaVIM)和10种mcr (mcr-1 ~ mcr-10)基因的多重重组酶聚合酶扩增技术。
质粒编码碳青霉烯酶和移动粘菌素耐药基因(mcr)的出现对控制多重耐药革兰氏阴性菌的传播提出了重大挑战。解决这一问题需要开发快速、准确和具有成本效益的基因检测工具。本研究首次报道了三种多重重组酶聚合酶扩增(RPA)方法,每种方法均用于检测五种耐药基因:碳青霉烯酶(blaKPC、blaNDM、blaoxa -48样、blaIMP和blaVIM)、mcr-1至mcr-5和mcr-6至mcr-10。使用琼脂糖凝胶电泳,所有15个靶基因都被三种检测方法成功扩增,证明了这些检测方法与快速报告平台集成的潜力。为了提高其适用性,将该检测方法与SYBR®Green I联合检测所有15个靶基因,并与侧流免疫分析法(LFIAs)联合检测两种碳青霉烯酶(blaNDM和blaoxa -48样)和两种mcr基因(mcr-1和mcr-3)基因。特异性测试表明,RPA-SYBR®Green I和RPA-LFIAs在靶基因之间不产生交叉反应。所有基因的RPA-SYBR®Green I的检出限为2 × 100 ~ 2 × 102 CFU/reaction, RPA-LFIAs的检出限为2 × 100 ~ 2 × 103 CFU/reaction。开发的RPA-SYBR®Green I和RPA-LFIAs成功地从阳性血液培养瓶中检测出15个和4个靶基因。这些检测方法为即时检测提供了一种很有前途的方法。为抗生素耐药性监测提供了宝贵的工具,并为有效的抗生素干预提供了及时的指导。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
21.60
自引率
0.90%
发文量
176
审稿时长
36 days
期刊介绍:
The International Journal of Antimicrobial Agents is a peer-reviewed publication offering comprehensive and current reference information on the physical, pharmacological, in vitro, and clinical properties of individual antimicrobial agents, covering antiviral, antiparasitic, antibacterial, and antifungal agents. The journal not only communicates new trends and developments through authoritative review articles but also addresses the critical issue of antimicrobial resistance, both in hospital and community settings. Published content includes solicited reviews by leading experts and high-quality original research papers in the specified fields.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


