Adjuvant Chemotherapy in Early Triple-Negative Breast Cancer (T1a-c N0M0): A Systematic Review and Meta-Analysis
IF 2.5
3区 医学
Q2 ONCOLOGY
引用次数: 0
Abstract
Triple-negative breast cancer (TNBC) is an aggressive subtype with limited treatment options and a high risk of early metastasis. While adjuvant chemotherapy (AdjCT) is standard for TNBC, its benefit in small, node-negative tumors (T1a, T1b, T1c) remains uncertain. This meta-analysis evaluates the survival impact of AdjCT in early-stage TNBC, focusing on overall survival (OS), breast cancer-specific survival (BCSS), and disease-free survival (DFS).
A comprehensive search was conducted in PubMed, the Cochrane Library, and Embase, yielding a total of 2089 studies. Hazard ratios (HR) and odds ratios (OR) were estimated using a random-effects model with 95% confidence intervals (CI). Heterogeneity was assessed with I², considering P < .05 and I² > 25% as significant. Our meta-analysis included 12 cohort studies, comprising a total of 48,371 patients, of whom 32,341 were included in the AdjCT group. AdjCT improved OS overall (HR: 0.75; 95% CI, 0.64-0.88; P < .001; I² = 83%), in T1b (HR: 0.73; P = .013; I² = 74%) and T1c tumors (HR: 0.73; P = .19; I² = 89%), but not in T1a (HR: 0.88; P = 0.646). BCSS showed a significant overall benefit (HR: 0.21; P = .042; I² = 98%), but no difference in T1a, T1b, or T1c subgroups. AdjCT also significantly improved 5-year DFS (OR: 2.08; 95% CI, 1.32-3.29; P = .002; I² = 0%). AdjCT improves OS in early-stage TNBC, particularly in T1b and T1c tumors, while significantly enhancing BCSS and DFS. No survival benefit was observed in T1a tumors, emphasizing the need for stage-based AdjCT decisions.
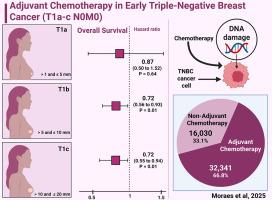
早期三阴性乳腺癌的辅助化疗(T1a-c N0M0):系统回顾和荟萃分析。
三阴性乳腺癌(TNBC)是一种侵袭性亚型,治疗选择有限,早期转移风险高。虽然辅助化疗(AdjCT)是TNBC的标准治疗方案,但其在小的淋巴结阴性肿瘤(T1a, T1b, T1c)中的益处仍不确定。该荟萃分析评估了AdjCT对早期TNBC患者的生存影响,重点关注总生存期(OS)、乳腺癌特异性生存期(BCSS)和无病生存期(DFS)。在PubMed、Cochrane图书馆和Embase中进行了全面的搜索,总共产生了2089项研究。使用随机效应模型估计风险比(HR)和优势比(OR),置信区间为95%。异质性以I²评估,认为P < 0.05和I²> 25%为显著性。我们的荟萃分析包括12项队列研究,共包括48,371例患者,其中32,341例纳入了AdjCT组。adct改善OS总体(HR: 0.75;95% ci, 0.64-0.88;P < .001;I²= 83%),T1b (HR: 0.73;P = 0.013;I²= 74%)和T1c肿瘤(HR: 0.73;P = .19;I²= 89%),但在T1a中没有(HR: 0.88;P = 0.646)。BCSS显示出显著的总体效益(HR: 0.21;P = 0.042;I²= 98%),但T1a、T1b或T1c亚组无差异。AdjCT也显著改善了5年DFS (OR: 2.08;95% ci, 1.32-3.29;P = .002;I²= 0%)。AdjCT改善早期TNBC的OS,尤其是T1b和T1c肿瘤,同时显著提高BCSS和DFS。在T1a肿瘤中没有观察到生存获益,强调了基于分期的AdjCT决定的必要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Clinical breast cancer
医学-肿瘤学
CiteScore
5.40
自引率
3.20%
发文量
174
审稿时长
48 days
期刊介绍:
Clinical Breast Cancer is a peer-reviewed bimonthly journal that publishes original articles describing various aspects of clinical and translational research of breast cancer. Clinical Breast Cancer is devoted to articles on detection, diagnosis, prevention, and treatment of breast cancer. The main emphasis is on recent scientific developments in all areas related to breast cancer. Specific areas of interest include clinical research reports from various therapeutic modalities, cancer genetics, drug sensitivity and resistance, novel imaging, tumor genomics, biomarkers, and chemoprevention strategies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


