Calycosin-7-O-β-D-glucoside alleviates nonalcoholic fatty liver disease by regulating short-chain fatty acids metabolism and metabolic regulators
IF 4.9
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Nonalcoholic fatty liver disease (NAFLD) is a metabolic stress liver injury disease caused by excessive fat deposition in hepatocytes. Astragalus memeranaceus Fisch., mainly consists of flavonoids, is considered to have good liver protection effects. This study aimed to explore the active ingredients and possible mechanisms of Astragalus flavone in the treatment of NAFLD. Network pharmacology and molecular docking were used to screen the active ingredients and targets of Astragalus flavone in the treatment of NAFLD, and were verified in vivo high-fat diet-induced mouse models. There were 89 predicted targets of 10 main compounds of Astragalus flavone in treating NAFLD, and the core genes involved PPAR-γ. Molecular docking results showed that all the 10 main components of Astragalus flavone had a strong binding activity with PPAR-γ, among which CG had the strongest binding ability. In vivo results showed that CG inhibited obesity, reduced the disorder of glucose and lipid metabolism, alleviated liver function injury and lipid accumulation, and improved the oxidative stress and inflammatory response of NAFLD mice, playing a positive role in the treatment of NAFLD. In addition, CG promoted the expression of AMPK, PPAR-γ, and Nrf2, inhibited the expressions of NF-кB, and regulated the metabolism of short-chain fatty acids (SCFAs) in NAFLD. In conclusion, CG, the main active component of Astragalus flavone, exerts a positive therapeutic effect on NAFLD, which is related to CG altering various energy sensors and metabolic regulators (PPAR-γ, AMPK, NF-кB, and Nrf2) that contribute to changes in intestinal SCFAs.
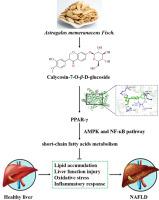
毛蕊异黄酮-7- o -β- d -葡萄糖苷通过调节短链脂肪酸代谢和代谢调节剂缓解非酒精性脂肪肝。
非酒精性脂肪性肝病(NAFLD)是一种由肝细胞脂肪过度沉积引起的代谢性应激性肝损伤疾病。黄芪。,主要由类黄酮组成,被认为具有良好的护肝作用。本研究旨在探讨黄芪黄酮治疗NAFLD的有效成分及可能机制。采用网络药理学和分子对接技术筛选黄芪黄酮治疗NAFLD的有效成分和靶点,并在体内高脂饮食诱导小鼠模型中进行验证。黄芪黄酮10种主要化合物治疗NAFLD的预测靶点达89个,核心基因均与PPAR-γ有关。分子对接结果表明,黄芪黄酮的10种主要成分均与PPAR-γ具有较强的结合活性,其中以CG结合能力最强。体内实验结果显示,CG抑制肥胖,减轻糖脂代谢紊乱,减轻肝功能损伤和脂质积累,改善NAFLD小鼠氧化应激和炎症反应,对NAFLD的治疗有积极作用。此外,CG可促进AMPK、PPAR-γ和Nrf2的表达,抑制NF-кB的表达,调节NAFLD中短链脂肪酸(SCFAs)的代谢。综上所述,黄芪黄酮的主要活性成分CG对NAFLD具有积极的治疗作用,其作用机制与CG改变肠道scfa变化的各种能量传感器和代谢调节因子(PPAR-γ、AMPK、NF-кB和Nrf2)有关。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Nutritional Biochemistry
医学-生化与分子生物学
CiteScore
9.50
自引率
3.60%
发文量
237
审稿时长
68 days
期刊介绍:
Devoted to advancements in nutritional sciences, The Journal of Nutritional Biochemistry presents experimental nutrition research as it relates to: biochemistry, molecular biology, toxicology, or physiology.
Rigorous reviews by an international editorial board of distinguished scientists ensure publication of the most current and key research being conducted in nutrition at the cellular, animal and human level. In addition to its monthly features of critical reviews and research articles, The Journal of Nutritional Biochemistry also periodically publishes emerging issues, experimental methods, and other types of articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


