Adaptive neofunctionalization of ancestral CYP6SN2vB following duplication contributes to abamectin resistance in Chilo suppressalis Walker
IF 3.7
2区 农林科学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Abamectin serves as a key alternative for managing diamide-resistant Chilo suppressalis. However, resistance to abamectin itself presents a significant challenge to this pest control, and its genetic basis remains poorly understood. In this study, we report an increase in field resistance to abamectin in populations of C. suppressalis and provide evidence for a cytochrome P450-mediated metabolic resistance mechanism, supported by enzyme activity assays and synergist bioassays. Transcriptomic profiling analysis revealed six candidate P450 genes, among which the duplicated gene CYP6SN2 showed a strong correlation with resistance. Variant CYP6SN2vB was highly expressed in detoxification tissues and mainly produced isoforms with a short 3′-UTR. Functional validation in transgenic Drosophila melanogaster confirmed that overexpression of CYP6SN2vB conferred 2.4- and 1.9-fold resistance to abamectin and emamectin benzoate, whereas CYP6SN2vA had no detectable effect. Molecular docking further revealed that CYP6SN2vB could potentially hydroxylate abamectin via interactions near oxygen-binding motifs. In contrast, the inward-folded substrate recognition site 1 region of CYP6SN2vA compresses the ligand-binding cavity, precluding abamectin interaction. These findings suggest neofunctionalization of CYP6SN2vB following duplication and provide valuable insights into the molecular basis of abamectin resistance in C. suppressalis, with implications for resistance management.
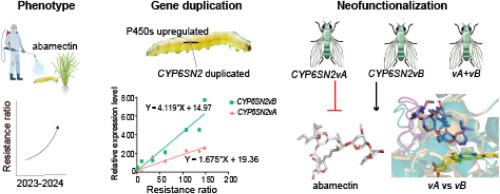
祖先CYP6SN2vB复制后的适应性新功能化有助于抑制Chilo Walker的阿维菌素耐药性。
阿维菌素是治疗抗二胺奇洛抑制菌的关键替代药物。然而,对阿维菌素的耐药性本身对这种害虫控制提出了重大挑战,其遗传基础仍然知之甚少。在这项研究中,我们报道了抑制C.种群对阿维菌素的田间抗性增加,并通过酶活性测定和增效剂生物测定为细胞色素p450介导的代谢抗性机制提供了证据。转录组学分析发现6个候选P450基因,其中重复基因CYP6SN2与抗性有较强的相关性。变异CYP6SN2vB在解毒组织中高表达,主要产生具有短3'-UTR的异构体。在转基因黑腹果蝇中的功能验证证实,CYP6SN2vB的过表达使其对阿维菌素和阿维菌素苯甲酸酯的抗性增加2.4倍和1.9倍,而CYP6SN2vA则没有可检测到的影响。分子对接进一步揭示CYP6SN2vB可能通过氧结合基序附近的相互作用使阿维菌素羟基化。相反,CYP6SN2vA向内折叠的底物识别位点1区域压缩了配体结合腔,阻止了阿维菌素的相互作用。这些发现提示了CYP6SN2vB在重复后的新功能,并为抑制C.阿维菌素耐药的分子基础提供了有价值的见解,对耐药管理具有指导意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.40
自引率
5.30%
发文量
105
审稿时长
40 days
期刊介绍:
This international journal publishes original contributions and mini-reviews in the fields of insect biochemistry and insect molecular biology. Main areas of interest are neurochemistry, hormone and pheromone biochemistry, enzymes and metabolism, hormone action and gene regulation, gene characterization and structure, pharmacology, immunology and cell and tissue culture. Papers on the biochemistry and molecular biology of other groups of arthropods are published if of general interest to the readership. Technique papers will be considered for publication if they significantly advance the field of insect biochemistry and molecular biology in the opinion of the Editors and Editorial Board.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


