Steering oxygen-centred radicals with ground-state ene-reductases for enantioselective intermolecular hydroalkoxylations
IF 44.6
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
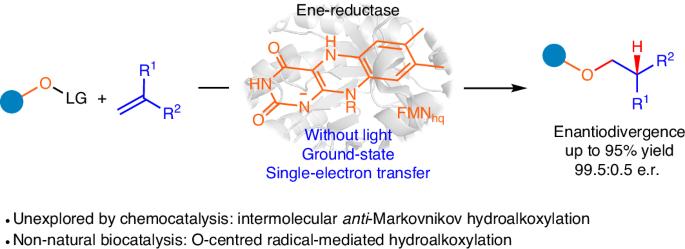
Enzymes are emerging as promising catalysts for selective radical transformations. However, non-natural radical-type enzymatic catalysis is currently limited to utilizing C-, N- and S-centred radical species. Alkoxy radicals are recognized as versatile intermediates with high reactivity, typically engaging in reactivity modes such as hydrogen atom transfer, β-scission processes and intramolecular addition to alkenes. Enantioselective intermolecular alkoxy radical addition to alkenes remained unknown. Here we develop a biocatalytic strategy based on engineered ene-reductases that facilitate the radical hydroalkoxylation of oxygen-centred radicals with alkenes. A single, ground-state ene-reductase adeptly controls the biocompatible generation of O-radicals, the follow-up intermolecular O-radical addition to alkenes and the final prochiral C-radical termination, achieving high chemo- and enantioselectivity (both enantiomers are obtained separately with different enzymes). Mechanistic experiments, including computational simulations, reveal that the radical enzymatic reaction initiates via a ground-state single-electron transfer and elucidate the origins of enantiodiscrimination of the overall reaction. The intermolecular addition of O-centred radicals to alkenes is a challenging endeavour in synthetic chemistry. Now ene-reductases are used to tame reactive O-radicals for intermolecular and enantioselective radical hydroalkoxylation involving a ground-state single-electron radical mechanism.

引导氧中心自由基与基态烯还原酶对映选择性分子间氢烷氧基化
酶正在成为选择性自由基转化的有前途的催化剂。然而,非天然自由基型酶催化目前仅限于利用C-、N-和s -中心自由基。烷氧基自由基是公认的具有高反应活性的多用途中间体,通常参与氢原子转移、β-裂解过程和烯烃分子内加成等反应模式。分子间烷氧基自由基加成烯烃的对映选择性尚不清楚。在这里,我们开发了一种基于工程烯还原酶的生物催化策略,促进氧中心自由基与烯烃的氢烷氧基化。单一的基态烯还原酶熟练地控制了o自由基的生物相容性生成,随后的分子间o自由基加入烯烃以及最终的前手性c自由基终止,实现了高的化学和对映体选择性(两种对映体分别由不同的酶获得)。包括计算模拟在内的机械实验揭示了自由基酶反应是通过基态单电子转移开始的,并阐明了整个反应对映体辨别的起源。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


