Transglutaminase 2 modulates inflammatory angiogenesis via vascular endothelial growth factor receptor 2 pathway in inflammatory bowel disease
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Objectives
Immune-driven inflammatory angiogenesis is a crucial component in the pathogenesis of inflammatory bowel disease (IBD). Nevertheless, the underlying mechanisms are still poorly understood. This study aims to investigate the role of Transglutaminase 2 (TGM2) in inflammatory angiogenesis in IBD and its potential as a therapeutic target and biomarker.Methods
We performed an RNA-seq analysis integrated single-cell transcriptomic profiling on IBD biopsies to identify dysregulated genes. Additionally, we explored TGM2 contribution to angiogenesis and colitis under in vitro and in vivo conditions in Tgm2 knockout mouse and human intestinal microvascular endothelial cells (HIMECs). Serum TGM2 levels were measured by Enzyme-Linked Immunosorbent Assay.Results
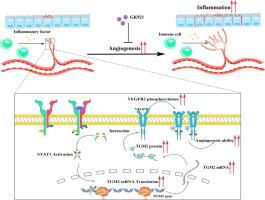
TGM2 expression was significantly upregulated in intestinal endothelial cells of IBD patients and colitis models. Tgm2 knockout and its inhibitor significantly attenuated intestinal colitis and angiogenesis in mice. In vitro, knockdown of TGM2 significantly suppressed tube formation, migration, and invasion of HIMECs. Mechanistically, inflammation-induced STAT1 activation promoted TGM2 expression, which subsequently interacted with vascular endothelial growth factor receptor 2 (VEGFR2) to drive its phosphorylation (Tyr1059, Tyr1214) and inflammatory angiogenesis. In patients of Crohn’s Disease, serum TGM2 concentrations exhibited high diagnostic accuracy (AUC = 0.862) for assessing endoscopic activity.Conclusion
Our findings underscore the critical role of STAT1-TGM2-VEGFR2 axis in regulating angiogenesis during intestinal inflammation, suggesting that targeting TGM2 as a viable therapeutic candidate for vascular remodeling in chronic intestinal inflammation.
转谷氨酰胺酶2通过血管内皮生长因子受体2途径调节炎症性肠病中的炎症血管生成
目的免疫驱动的炎症性血管生成是炎症性肠病(IBD)发病机制的重要组成部分。然而,人们对其潜在机制仍知之甚少。本研究旨在探讨转谷氨酰胺酶2 (TGM2)在IBD炎症血管生成中的作用及其作为治疗靶点和生物标志物的潜力。方法我们对IBD活检组织进行了RNA-seq分析和单细胞转录组分析,以鉴定失调基因。此外,我们探讨了TGM2基因敲除小鼠和人肠道微血管内皮细胞(HIMECs)在体外和体内条件下对血管生成和结肠炎的贡献。采用酶联免疫吸附法测定血清TGM2水平。结果stgm2在IBD患者和结肠炎模型肠内皮细胞中的表达明显上调。Tgm2敲除及其抑制剂可显著减轻小鼠肠道结肠炎和血管生成。在体外,敲低TGM2可显著抑制himec的小管形成、迁移和侵袭。在机制上,炎症诱导的STAT1激活促进了TGM2的表达,TGM2随后与血管内皮生长因子受体2 (VEGFR2)相互作用,驱动其磷酸化(Tyr1059, Tyr1214)和炎症性血管生成。在克罗恩病患者中,血清TGM2浓度在评估内镜活动方面表现出很高的诊断准确性(AUC = 0.862)。我们的研究结果强调了STAT1-TGM2-VEGFR2轴在调节肠道炎症期间血管生成中的关键作用,表明靶向TGM2是慢性肠道炎症血管重构的可行治疗候选。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


