Large scale laboratory evolution uncovers clinically relevant collateral antibiotic sensitivity
IF 4.6
2区 医学
Q1 INFECTIOUS DISEASES
International Journal of Antimicrobial Agents
Pub Date : 2025-07-02
DOI:10.1016/j.ijantimicag.2025.107564
引用次数: 0
Abstract
The increasing prevalence of antibiotic resistance is a critical challenge, necessitating the development of strategies to mitigate the evolution of resistance. Collateral sensitivity (CS)-based sequential therapies have been proposed to mitigate resistance evolution.
However, the evolutionary repeatability of CS across different experimental conditions and its clinical relevance remain underexplored, hindering its potential for translation into clinical practice. Here, we evolve 20–24 lineages of E. coli against tigecycline (TIG) and piperacillin (PIP), antibiotics suggested to produce CS, through three separate laboratory adaptive evolution (ALE) platforms to test for the robustness of CS interactions and the effect of the choice of ALE on CS evolution. We generate over 130 resistant mutants and 540 resistance and collateral sensitivity measurements to identify a CS relationship between TIG and polymyxin B (POL) that is highly repeatable across all the ALEs tested, suggesting that this CS interaction is preserved across different evolution microenvironments. We determine the mechanism of this novel CS by showing that cells resistant to TIG deactivate the Lon protease and overproduce negatively charged exopolysaccharides, which in turn attracts the polycationic POL and renders cells hypersensitive to the drug. We find that this CS relationship is present in a clinical dataset of over 750 uropathogenic MDR E. coli isolates, and show that the soft agar gradient evolution (SAGE) platform best predicts collateral effects (CS, neutrality or cross resistance) in this dataset. Our study provides a framework for identifying robust CS with clinical implications that can reduce the emergence of resistance to our existing antibiotics.
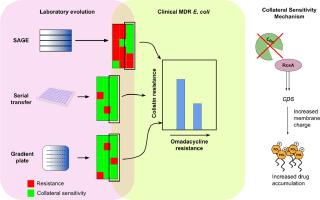
大规模的实验室进化揭示了临床相关的侧支抗生素敏感性。
日益普遍的抗生素耐药性是一个严峻的挑战,需要制定策略来减缓耐药性的演变。基于侧枝敏感性(CS)的序贯疗法已被提出以减轻耐药性的演变。然而,CS在不同实验条件下的进化可重复性及其临床相关性仍未得到充分探索,阻碍了其转化为临床实践的潜力。在这里,我们通过三个独立的实验室适应进化(ALE)平台,进化了20-24个大肠杆菌菌株,以对抗替加环素(TIG)和哌拉西林(PIP),这两种抗生素被认为可以产生CS,以测试CS相互作用的稳健性以及ALE选择对CS进化的影响。我们产生了超过130个耐药突变体和540个耐药和附加敏感性测量,以确定TIG和多粘菌素B (POL)之间的CS关系,该关系在所有测试的ALEs中都是高度可重复的,这表明这种CS相互作用在不同的进化微环境中保持不变。我们通过证明抗TIG的细胞使Lon蛋白酶失活并过量产生带负电荷的胞外多糖,从而吸引多阳离子POL并使细胞对药物过敏,从而确定了这种新型CS的机制。我们发现这种CS关系存在于超过750个尿路致病性MDR大肠杆菌分离株的临床数据集中,并表明软琼脂梯度进化(SAGE)平台最好地预测该数据集中的附带效应(CS,中性或交叉抗性)。我们的研究为确定具有临床意义的强大CS提供了一个框架,可以减少对现有抗生素的耐药性的出现。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
21.60
自引率
0.90%
发文量
176
审稿时长
36 days
期刊介绍:
The International Journal of Antimicrobial Agents is a peer-reviewed publication offering comprehensive and current reference information on the physical, pharmacological, in vitro, and clinical properties of individual antimicrobial agents, covering antiviral, antiparasitic, antibacterial, and antifungal agents. The journal not only communicates new trends and developments through authoritative review articles but also addresses the critical issue of antimicrobial resistance, both in hospital and community settings. Published content includes solicited reviews by leading experts and high-quality original research papers in the specified fields.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


