Human serum lipids affect Staphylococcus aureus sensitivity to phage infection
IF 4.6
2区 医学
Q1 INFECTIOUS DISEASES
International Journal of Antimicrobial Agents
Pub Date : 2025-07-03
DOI:10.1016/j.ijantimicag.2025.107568
引用次数: 0
Abstract
Objectives
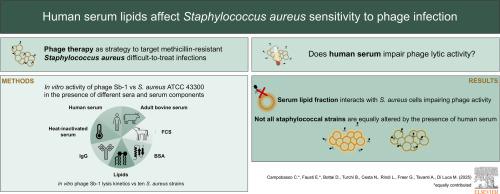
To explore phage therapy as an alternative for targeting multidrug-resistant bacterial strains, it is crucial to study phage-bacteria interaction under conditions that mimic in vivo environments. Recent studies have demonstrated that staphylococcal phage activity can be significantly hindered by human blood components, including plasma and serum. This study aimed to identify serum components responsible for phage inhibition and assess whether this effect occurred across multiple Staphylococcus aureus strains.
Methods
Phage Sb-1 activity against S. aureus ATCC 43300 was tested by pre-incubating or co-incubating bacteria and phages with 30% (v/v) heat-inactivated (56 °C and 80 °C) human, bovine, or foetal calf serum, IgG-depleted or delipidated serum, and BSA. Bacteria were incubated with serum before phage exposure, followed by washing with 0.1% Triton X-100. Additionally, growth kinetics of ten S. aureus strains incubated with or without Sb-1 were assessed over 24 hours in the presence of human serum.
Results
We found that adult human serum completely impairs phage infectivity due to interactions between serum components and bacterial cells rather than direct phage neutralisation. Albumin, IgG, and thermolabile components were demonstrated not to significantly contribute to the inhibitory effect, whereas lipids were identified as playing a key role. Furthermore, the sensitivity of different bacterial strains to phages was shown to be differentially affected by the presence of serum, as two of the strains tested remained susceptible to lysis despite serum exposure.
Conclusions
Taken together, our findings suggest that serum lipid fraction impacts phage infectivity in a strain-specific manner, highlighting the need for tailored approaches for phage therapy.
人血脂影响金黄色葡萄球菌对噬菌体感染的敏感性。
为了探索噬菌体治疗作为靶向多药耐药菌株的替代方案,在模拟体内环境的条件下研究噬菌体-细菌相互作用至关重要。最近的研究表明,葡萄球菌噬菌体活性可被人体血液成分(包括血浆和血清)显著阻碍。本研究旨在鉴定负责噬菌体抑制的血清成分,并评估这种作用是否发生在多种金黄色葡萄球菌菌株中。通过将细菌和噬菌体与30% (v/v)热灭活(56°C和80°C)的人、牛或胎牛血清、igg耗尽或降解血清和BSA一起预孵育或共孵育,检测噬菌体Sb-1对金黄色葡萄球菌ATCC 43300的活性。细菌在噬菌体暴露前与血清孵育,然后用0.1% Triton X-100洗涤。此外,在人血清存在的情况下,对10株金黄色葡萄球菌加Sb-1或不加Sb-1培养24小时的生长动力学进行了评估。我们发现,由于血清成分与细菌细胞之间的相互作用,成人血清完全损害了噬菌体的感染性,而不是直接中和噬菌体。白蛋白、IgG和耐热性成分被证明对抑制作用没有显著贡献,而脂质被确定为起关键作用。此外,不同菌株对噬菌体的敏感性受到血清存在的不同影响,因为尽管血清暴露,两种被测试的菌株仍然对裂解敏感。综上所述,我们的研究结果表明,血清脂质分数以菌株特异性的方式影响噬菌体的感染性,这突出了噬菌体治疗的量身定制方法的必要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
21.60
自引率
0.90%
发文量
176
审稿时长
36 days
期刊介绍:
The International Journal of Antimicrobial Agents is a peer-reviewed publication offering comprehensive and current reference information on the physical, pharmacological, in vitro, and clinical properties of individual antimicrobial agents, covering antiviral, antiparasitic, antibacterial, and antifungal agents. The journal not only communicates new trends and developments through authoritative review articles but also addresses the critical issue of antimicrobial resistance, both in hospital and community settings. Published content includes solicited reviews by leading experts and high-quality original research papers in the specified fields.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


