A patient-derived organoid model captures fetal-like plasticity in colorectal cancer
IF 25.9
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
Phenotypic plasticity is a hallmark feature driving cancer progression, metastasis, and therapy resistance. Fetal-like transcriptional programs have been increasingly implicated in promoting plastic cell states, yet their roles remain difficult to study due to limitations of existing culture models. Here, we establish a chemically defined patient-derived organoid system that enables long-term expansion of colorectal cancer (CRC) cells while preserving fetal-like features associated with phenotypic plasticity. Using this model, we identify an oncofetal state (OnFS) that is enriched in advanced tumors and linked to key features of plasticity, including epithelial-mesenchymal plasticity, as well as increased metastasis and treatment resistance. Mechanistically, we show that FGF2-AP-1 signaling maintains the OnFS program and associated phenotypic plasticity in CRC. This model offers a powerful platform for studying the fetal-like features underlying cancer cell plasticity and their role in tumor progression and treatment resistance in CRC.
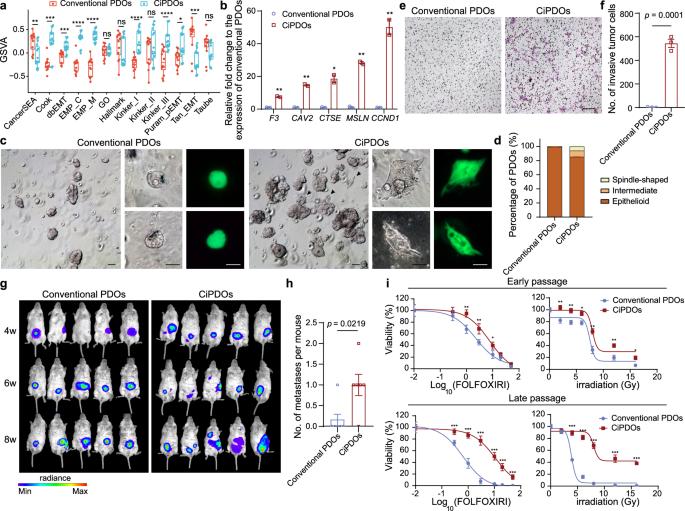
患者来源的类器官模型捕获结肠直肠癌胎儿样可塑性。
表型可塑性是驱动癌症进展、转移和治疗抵抗的标志性特征。胎儿样转录程序越来越多地参与促进可塑性细胞状态,但由于现有培养模型的限制,它们的作用仍然难以研究。在这里,我们建立了一个化学定义的患者来源的类器官系统,使结直肠癌(CRC)细胞能够长期扩张,同时保留与表型可塑性相关的胎儿样特征。使用该模型,我们确定了在晚期肿瘤中丰富的癌胎状态(OnFS),并与可塑性的关键特征相关,包括上皮-间充质可塑性,以及增加的转移和治疗耐药性。在机制上,我们发现FGF2-AP-1信号维持了CRC的OnFS程序和相关的表型可塑性。该模型为研究CRC中癌细胞可塑性的胎儿样特征及其在肿瘤进展和治疗耐药中的作用提供了一个强大的平台。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Research
生物-细胞生物学
CiteScore
53.90
自引率
0.70%
发文量
2420
审稿时长
2.3 months
期刊介绍:
Cell Research (CR) is an international journal published by Springer Nature in partnership with the Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences (CAS). It focuses on publishing original research articles and reviews in various areas of life sciences, particularly those related to molecular and cell biology. The journal covers a broad range of topics including cell growth, differentiation, and apoptosis; signal transduction; stem cell biology and development; chromatin, epigenetics, and transcription; RNA biology; structural and molecular biology; cancer biology and metabolism; immunity and molecular pathogenesis; molecular and cellular neuroscience; plant molecular and cell biology; and omics, system biology, and synthetic biology. CR is recognized as China's best international journal in life sciences and is part of Springer Nature's prestigious family of Molecular Cell Biology journals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


