Biomass char gasification for hydrogen production: A thermodynamic equilibrium analysis
IF 7.7
2区 工程技术
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
Hydrogen can be produced from biomass via thermochemical conversion, either through direct gasification of raw biomass or a two-step process involving pyrolysis to generate char, followed by char gasification. The latter process reduces tar contamination in syngas and improves gasification efficiency. Chemical equilibrium modeling of char gasification provides valuable estimates of ideal operating conditions, serving as an effective tool for gasifier design and optimization. The analysis focuses on the effects of process parameters on hydrogen yield and efficiency, as well as the determination of optimal thermodynamic conditions for hydrogen production. The chemical composition of wood char at different pyrolysis temperatures was examined using literature data. Char gasification in steam was predicted using Gibbs energy minimization, identifying up to 122 product species. The optimal conditions were identified within a temperature range of 900–1300 °C and a steam-to-char molar ratio () of 0.6–0.9, yielding a hydrogen molar fraction of 0.53 ± 0.01. Under these conditions, the primary gases produced were (0.53) and (0.45). Further optimization of process efficiency suggested that setting the gasification temperature to 800 °C and the steam-to-char molar ratio to 1.3 could achieve a hydrogen molar fraction of 0.5 while maintaining a process efficiency of 55%.
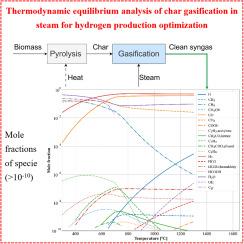
生物质炭气化制氢:热力学平衡分析
氢可以通过热化学转化从生物质中产生,既可以通过原料生物质的直接气化,也可以通过热解生成木炭的两步过程,然后再进行木炭气化。后一种工艺减少了合成气中的焦油污染,提高了气化效率。炭气化的化学平衡模型提供了理想操作条件的有价值的估计,作为气化炉设计和优化的有效工具。重点分析了工艺参数对产氢率和效率的影响,并确定了产氢的最佳热力学条件。利用文献资料研究了不同热解温度下木炭的化学成分。利用吉布斯能量最小化法预测了蒸汽中的焦炭气化,确定了多达122种产物。在900 ~ 1300℃的温度范围和0.6 ~ 0.9的蒸汽炭摩尔比(m)范围内,得到的氢摩尔分数为0.53±0.01。在此条件下,主要气体为H2(0.53)和CO(0.45)。进一步优化工艺效率表明,当气化温度为800℃,汽炭摩尔比为1.3时,氢气摩尔分数为0.5,工艺效率为55%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Fuel Processing Technology
工程技术-工程:化工
CiteScore
13.20
自引率
9.30%
发文量
398
审稿时长
26 days
期刊介绍:
Fuel Processing Technology (FPT) deals with the scientific and technological aspects of converting fossil and renewable resources to clean fuels, value-added chemicals, fuel-related advanced carbon materials and by-products. In addition to the traditional non-nuclear fossil fuels, biomass and wastes, papers on the integration of renewables such as solar and wind energy and energy storage into the fuel processing processes, as well as papers on the production and conversion of non-carbon-containing fuels such as hydrogen and ammonia, are also welcome. While chemical conversion is emphasized, papers on advanced physical conversion processes are also considered for publication in FPT. Papers on the fundamental aspects of fuel structure and properties will also be considered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


