Innovative sulfation strategy for efficient recovery of rare earth elements from spent fluorescent lamp powder
IF 10.9
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
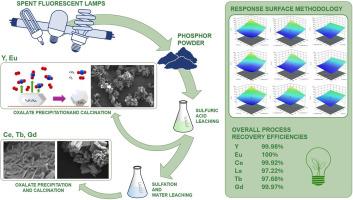
The recovery of rare earth elements (REEs) from spent fluorescent lamp phosphors is essential for resource sustainability and circular hydrometallurgy. While yttrium (Y) and europium (Eu) can be recovered through simple acid leaching, extracting cerium (Ce), lanthanum (La), and terbium (Tb) from green phosphors is challenging due to their resistance to dissolution. This study presents a novel sulfation-based process at relatively low temperatures to selectively recover REEs, particularly Tb, while minimizing energy consumption and environmental impact by preventing sulfuric acid and mercury evaporation.
Initially, sulfuric acid leaching selectively recovered most of Y and Eu, leaving other REEs in the solid residue. These elements were then precipitated as a nearly pure REE oxalate mixture (>99 % purity). The effects of sulfation time, temperature, and acid-to-powder weight ratio were optimized using Response Surface Methodology (RSM). Sulfation at 150 °C for 5 h enabled the recovery of nearly 100 % Ce and Gd, 98.01 % La, and 98.15 % Tb through water leaching. The mixed oxalate precipitate, enriched in La, Ce, and Tb, exhibited >98 % purity. SEM analysis revealed the formation of flower-like mixed REE oxides after calcination.
This process achieved high recovery efficiencies of 99.98 % Y, 100 % Eu, 99.92 % Ce, 97.22 % La, 97.68 % Tb, and 99.97 % Gd. By using only sulfuric and oxalic acid, it aligns with sustainable hydrometallurgy, reducing chemical diversity and enabling acid regeneration. This study provides an efficient, environmentally friendly approach for REE recovery from phosphor waste.
从废荧光灯粉中高效回收稀土元素的创新磺化策略
从废荧光灯荧光粉中回收稀土元素对资源可持续性和循环湿法冶金至关重要。虽然钇(Y)和铕(Eu)可以通过简单的酸浸回收,但从绿色荧光粉中提取铈(Ce)、镧(La)和铽(Tb)是具有挑战性的,因为它们不易溶解。本研究提出了一种在相对低温下选择性回收稀土,特别是Tb的新型硫化工艺,同时通过防止硫酸和汞蒸发,最大限度地减少能源消耗和对环境的影响。最初,硫酸浸出选择性地回收了大部分Y和Eu,其余稀土元素留在固体残渣中。然后将这些元素沉淀成几乎纯的REE草酸盐混合物(纯度为99%)。采用响应面法(RSM)对磺化时间、温度、酸粉比等因素的影响进行了优化。在150°C下硫化5 h,通过水浸可以回收近100%的Ce和Gd, 98.01%的La和98.15%的Tb。混合草酸沉淀,富含La, Ce和Tb,纯度为98%。SEM分析表明,煅烧后形成花状混合稀土氧化物。该工艺回收率为99.98% Y、100% Eu、99.92% Ce、97.22% La、97.68% Tb、99.97% Gd。通过仅使用硫酸和草酸,它与可持续湿法冶金相一致,减少了化学多样性并实现了酸再生。本研究为从磷光废料中回收稀土元素提供了一种高效、环保的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Resources Conservation and Recycling
环境科学-工程:环境
CiteScore
22.90
自引率
6.10%
发文量
625
审稿时长
23 days
期刊介绍:
The journal Resources, Conservation & Recycling welcomes contributions from research, which consider sustainable management and conservation of resources. The journal prioritizes understanding the transformation processes crucial for transitioning toward more sustainable production and consumption systems. It highlights technological, economic, institutional, and policy aspects related to specific resource management practices such as conservation, recycling, and resource substitution, as well as broader strategies like improving resource productivity and restructuring production and consumption patterns.
Contributions may address regional, national, or international scales and can range from individual resources or technologies to entire sectors or systems. Authors are encouraged to explore scientific and methodological issues alongside practical, environmental, and economic implications. However, manuscripts focusing solely on laboratory experiments without discussing their broader implications will not be considered for publication in the journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


