Cu(I)-Catalyzed [4 + 1] Cycloaddition of o-Quinone Methides with Terminal Alkynes: A Rapid Synthesis of 2,3-Disubstituted Benzofurans
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A Cu(I)-catalyzed [4 + 1] cycloaddition reaction between o-quinone methides and terminal alkynes has been developed, demonstrating good compatibility with both stable and in situ generated o-quinone methides. This methodology offers several significant advantages, including operational simplicity, wide substrate compatibility, and the ability to access synthetically useful 2,3-disubstituted benzofuran compounds in generally good to high yields (44 examples, up to 91% yield).
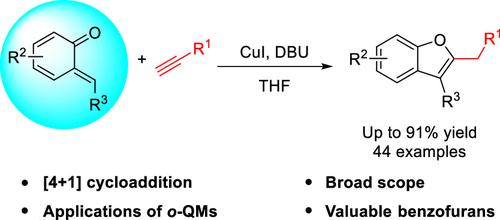
Cu(I)催化[4 + 1]邻醌与末端炔的环加成反应:快速合成2,3-二取代苯并呋喃。
建立了Cu(I)催化的邻醌类化合物与末端炔之间的[4 + 1]环加成反应,与稳定的邻醌类化合物和原位生成的邻醌类化合物均具有良好的相容性。该方法具有几个显著的优点,包括操作简单,底物兼容性广,能够获得合成上有用的2,3-二取代苯并呋喃化合物,收率通常很高(44个例子,收率高达91%)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


