Inhalation toxicity of cellulose nanofibrils: A review of key findings and future directions
IF 6.5
Q1 CHEMISTRY, APPLIED
Carbohydrate Polymer Technologies and Applications
Pub Date : 2025-06-27
DOI:10.1016/j.carpta.2025.100913
引用次数: 0
Abstract
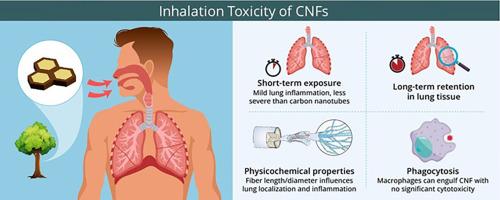
Cellulose nanofibrils (CNFs) have attracted increasing research attention as sustainable and biodegradable nanomaterials with applications across diverse industrial sectors, including packaging, agriculture, and biomedical engineering. However, owing to their ultrafine size and fibrous morphology, concerns have emerged regarding their potential inhalation toxicity and long-term health effects. This review examines recent studies addressing CNF-induced lung inflammation, pulmonary distribution, retention, clearance, phagocytosis by alveolar macrophages, and in vitro cytotoxicity. Key findings indicate that short-term exposure to CNFs generally induces mild pulmonary inflammation, which is less severe compared to that caused by other fibrous nanomaterials such as carbon nanotubes (CNTs). The fiber length and diameter of CNFs significantly influence their pulmonary distribution and the severity of inflammatory responses. CNFs exhibit prolonged retention in the lung tissue and are phagocytosed by alveolar macrophages, although no significant cytotoxicity has been observed in vitro. Biological impurities, including bacterial endotoxins, are often present in CNF suspensions and may influence toxicity outcomes; however, their exact contributions remain unclear. Due to variations in source materials and processing methods, CNFs require case-by-case inhalation toxicity assessment. This review also highlights the need for standardized characterization, advanced exposure models, and appropriate safety and regulatory measures for their responsible use.
纤维素纳米原纤维的吸入毒性:主要发现和未来发展方向的综述
纤维素纳米原纤维(CNFs)作为一种可持续和可生物降解的纳米材料,在包装、农业和生物医学工程等各个工业领域得到了越来越多的研究关注。然而,由于其超细尺寸和纤维形态,人们对其潜在的吸入毒性和长期健康影响感到担忧。本文综述了最近关于cnf诱导的肺部炎症、肺分布、滞留、清除、肺泡巨噬细胞吞噬和体外细胞毒性的研究。关键发现表明,短期暴露于CNFs通常会引起轻度肺部炎症,与碳纳米管(CNTs)等其他纤维纳米材料相比,这种炎症不那么严重。CNFs的纤维长度和直径显著影响其肺分布和炎症反应的严重程度。CNFs在肺组织中表现出长时间的滞留,并被肺泡巨噬细胞吞噬,尽管在体外没有观察到明显的细胞毒性。生物杂质,包括细菌内毒素,经常存在于CNF悬浮液中,并可能影响毒性结果;然而,他们的具体贡献仍不清楚。由于源材料和加工方法的差异,CNFs需要逐个评估吸入毒性。这篇综述还强调了标准化表征、先进的暴露模型和适当的安全和监管措施的必要性,以便负责任地使用它们。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


