Porous cobalt-containing poly(ionic liquid) catalyst for tandem desulfurization and selective catalytic oxidation
IF 7.5
1区 工程技术
Q2 ENERGY & FUELS
引用次数: 0
Abstract
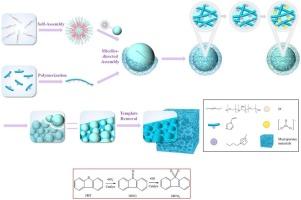
This study describes the synthesis of a porous polyvinyl imidazole cobalt chloride catalyst (denoted herein as pore-poly-[BVIM]CoCl3) and its subsequent application in a two-step extraction-catalytic oxidation process for the desulfurization of octane. The catalyst synthesis process employed a porogen (P123) and low stirring speeds, which significantly increased the specific surface area of the catalyst from 78.5 m2/g (no P123) to 1196 m2/g and provided an average pore size of 26.46 nm. The pore-poly-[BVIM]CoCl3 catalyst possessed uniformly distributed pores with cobalt ions dispersed throughout. The average molecular weight of the catalyst was 4090 g/mol, corresponding to 44 units of 3-butyl-1-vinylimidazolium cobalt chloride. Octane containing 500 ppm dibenzothiophene (DBT) was desulfurized in a two-step process, which involved the extraction of DBT into acetonitrile followed by the catalytic oxidation of DBT using the pore-poly-[BVIM]CoCl3 catalyst with peroxymonosulfate (PMS) as the oxidant. The desulfurization experiment was optimally performed using 20 mg of catalyst, 0.6 g of 20 wt% PMS solution, 2 g of acetonitrile (extraction solvent), a reaction temperature of 30 °C, and an initial sulfur content of 500 ppm. Under these conditions, >99 % desulfurization was achieved in 15 min. Moreover, the porous catalyst maintained a desulfurization rate of > 90 % after 7 cycles of use, demonstrating excellent durability and reusability. The oxidation mechanism of DBT was determined through GC–MS analysis and density functional theory (DFT) calculations: DBT is first oxidized by a sulfate radical to dibenzothiophene oxide (DBTO), which is then further oxidized by hydroxyl radicals to dibenzothiophene sulfone (DBTO2). The radical scavenger tert-butyl alcohol (TBA) inhibited hydroxyl radical attack in the second step, thereby stabilizing DBTO. This study’s findings demonstrate the potential of octane desulfurization and the mild catalytic oxidative synthesis of sulfur-containing organic compounds in the pharmaceutical sector.
用于串联脱硫和选择性催化氧化的多孔含钴多离子液体催化剂
本研究描述了多孔聚氯乙烯咪唑氯化钴催化剂(本文称多孔聚[BVIM]CoCl3)的合成及其在两步萃取-催化氧化法脱硫辛烷中的应用。催化剂的合成过程采用了多孔剂P123和低搅拌速度,使催化剂的比表面积从78.5 m2/g(无P123)显著提高到1196 m2/g,平均孔径为26.46 nm。多孔聚[BVIM]CoCl3催化剂具有均匀分布的孔洞,钴离子分散在各处。催化剂的平均分子量为4090g /mol,相当于44个单位的3-丁基-1-乙烯基咪唑氯化钴。采用两步法对含500 ppm二苯并噻吩(DBT)辛烷值进行了脱硫,即先将DBT萃取到乙腈中,再以过氧单硫酸根(PMS)为氧化剂,采用多孔聚[BVIM]CoCl3催化剂催化氧化DBT。脱硫实验的最佳条件为催化剂20 mg, 20% PMS溶液0.6 g,乙腈(萃取溶剂)2g,反应温度30℃,初始硫含量500 ppm。在此条件下,可在15 min内实现99%的脱硫,且多孔催化剂的脱硫率保持在经过7次循环使用后可达到90%,具有优异的耐用性和可重复使用性。通过GC-MS分析和密度泛函理论(DFT)计算确定DBT的氧化机理:DBT首先被硫酸盐自由基氧化为二苯并噻吩氧化物(DBTO),然后再被羟基自由基氧化为二苯并噻吩砜(DBTO2)。自由基清除剂叔丁醇(TBA)在第二步抑制羟基自由基的攻击,从而稳定DBTO。本研究的发现证明了辛烷值脱硫和含硫有机化合物的轻度催化氧化合成在制药领域的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Fuel
工程技术-工程:化工
CiteScore
12.80
自引率
20.30%
发文量
3506
审稿时长
64 days
期刊介绍:
The exploration of energy sources remains a critical matter of study. For the past nine decades, fuel has consistently held the forefront in primary research efforts within the field of energy science. This area of investigation encompasses a wide range of subjects, with a particular emphasis on emerging concerns like environmental factors and pollution.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


