Multimodal AI to forecast arrhythmic death in hypertrophic cardiomyopathy
IF 10.8
Q1 CARDIAC & CARDIOVASCULAR SYSTEMS
引用次数: 0
Abstract
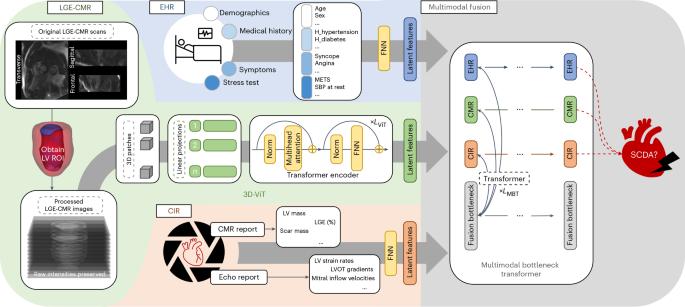
Sudden cardiac death from ventricular arrhythmias is a leading cause of mortality worldwide. Arrhythmic death prognostication is challenging in patients with hypertrophic cardiomyopathy (HCM), a setting where current clinical guidelines show low performance and inconsistent accuracy. Here, we present a deep learning approach, MAARS (Multimodal Artificial intelligence for ventricular Arrhythmia Risk Stratification), to forecast lethal arrhythmia events in patients with HCM by analyzing multimodal medical data. MAARS’ transformer-based neural networks learn from electronic health records, echocardiogram and radiology reports, and contrast-enhanced cardiac magnetic resonance images, the latter being a unique feature of this model. MAARS achieves an area under the curve of 0.89 (95% confidence interval (CI) 0.79–0.94) and 0.81 (95% CI 0.69–0.93) in internal and external cohorts and outperforms current clinical guidelines by 0.27–0.35 (internal) and 0.22–0.30 (external). In contrast to clinical guidelines, it demonstrates fairness across demographic subgroups. We interpret MAARS’ predictions on multiple levels to promote artificial intelligence transparency and derive risk factors warranting further investigation. Lai et al. present the machine learning model MAARS to predict arrhythmic sudden cardiac death from multimodal imaging and clinical data in patients with hypertrophic cardiomyopathy.
多模式人工智能预测肥厚性心肌病患者心律失常死亡。
室性心律失常引起的心源性猝死是世界范围内死亡的主要原因。肥厚性心肌病(HCM)患者的心律失常死亡预测具有挑战性,目前的临床指南表现出较低的性能和不一致的准确性。在这里,我们提出了一种深度学习方法MAARS(多模态人工智能室性心律失常风险分层),通过分析多模态医疗数据来预测HCM患者的致命心律失常事件。MAARS基于变压器的神经网络从电子健康记录、超声心动图和放射学报告以及对比增强心脏磁共振图像中学习,后者是该模型的独特功能。MAARS在内部和外部队列中的曲线下面积分别为0.89(95%可信区间(CI) 0.79-0.94)和0.81 (95% CI 0.69-0.93),比目前的临床指南高出0.27-0.35(内部)和0.22-0.30(外部)。与临床指南相比,它显示了跨人口亚组的公平性。我们在多个层面上解释MAARS的预测,以提高人工智能的透明度,并得出需要进一步调查的风险因素。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


