Reductive coupling of acrylamides and carbonyls via solvated electrons from excited-state acridyl radicals
IF 19.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The reductive coupling of carbonyls and alkenes is primarily promoted by super stoichiometric quantities of samarium diiodide (SmI2). This reductant requires fresh preparation under moisture-free anaerobic conditions for successful reactivity. Herein, we describe a method for reductively coupling acrylamides with unactivated ketones and aldehydes by employing acridine radicals as excited-state reductants. Ultrafast transient absorption spectroscopy experiments provide a clearer picture of the excited-state acridine radical and its mechanism of action, which most likely proceeds via the formation of solvated electrons in acetonitrile.

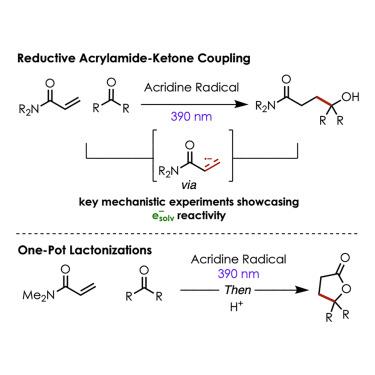
丙烯酰胺和羰基通过激发态吖啶基自由基溶剂化电子的还原偶联
羰基和烯烃的还原偶联主要是由超化学计量量的二碘化钐(SmI2)促进的。这种还原剂需要在无湿厌氧条件下新鲜制备才能成功反应。在此,我们描述了一种利用吖啶自由基作为激发态还原剂,将丙烯酰胺与未活化的酮和醛还原偶联的方法。超快瞬态吸收光谱实验提供了更清晰的激发态吖啶自由基及其作用机制的图像,这很可能是通过在乙腈中形成溶剂化电子进行的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


