Molecular Iodine‐Mediated Functionalization of α‐Carbonyl Sulfoxonium Ylides with Thiocyanates, Xanthates, and Dithiocarbamates
IF 2.7
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
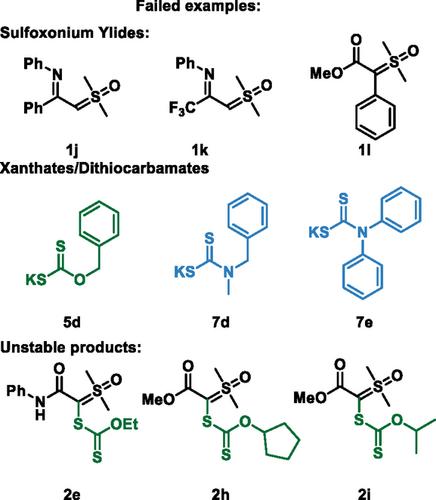
In this work, a straightforward, rapid, and cost‐effective protocol is presented for synthesizing three distinct classes of α‐functionalized sulfoxonium ylides—α‐xanthate, α‐thiocyanate, and α‐dithiocarbamate—via molecular iodine‐mediated reactions. This method demonstrates broad substrate tolerance, including heterocycles and steroids, delivering 25 examples with yields of up to 83%. Experimental studies revealed that iodine reacts with xanthates to form disulfides, which subsequently act as electrophiles for nucleophilic attack by the ylide.

硫氰酸盐、黄原酸盐和二硫代氨基甲酸盐介导的α -羰基亚砜酰化物分子功能化
在这项工作中,我们提出了一种简单、快速、经济有效的方案,通过分子碘介导的反应合成了三种不同类别的α‐功能化亚砜基化物——α‐黄药、α‐硫氰酸盐和α‐二硫代氨基甲酸盐。该方法具有广泛的底物耐受性,包括杂环化合物和类固醇,提供25个样品,产率高达83%。实验研究表明,碘与黄药反应形成二硫化物,二硫化物随后作为亲电试剂被叶利德亲核攻击。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.40
自引率
3.60%
发文量
752
审稿时长
1 months
期刊介绍:
The European Journal of Organic Chemistry (2019 ISI Impact Factor 2.889) publishes Full Papers, Communications, and Minireviews from the entire spectrum of synthetic organic, bioorganic and physical-organic chemistry. It is published on behalf of Chemistry Europe, an association of 16 European chemical societies.
The following journals have been merged to form two leading journals, the European Journal of Organic Chemistry and the European Journal of Inorganic Chemistry:
Liebigs Annalen
Bulletin des Sociétés Chimiques Belges
Bulletin de la Société Chimique de France
Gazzetta Chimica Italiana
Recueil des Travaux Chimiques des Pays-Bas
Anales de Química
Chimika Chronika
Revista Portuguesa de Química
ACH—Models in Chemistry
Polish Journal of Chemistry.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


