Quantitative Relationships between Physicochemical Properties of Organic Carbon from Coal Combustion and Heterogeneous Photooxidation of SO2 to Sulfates
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
Coal combustion significantly contributes to atmospheric organic carbon (OC), which can act as photosensitive sites for the conversion of SO2 to sulfates. Light can significantly enhance the sulfate formation by SO2 uptake on OC from coal combustion. These OC owned variable photo-reactivity, as shown by distinct SO2 uptake coefficients and sulfate production. It was confirmed that the photo-reactivity of OC towards SO2 greatly relied on its physicochemical properties, including hydrophilicity, light absorption capacity and •OH generation ability. The linear correlations of these properties with SO2 uptake and sulfate production have been well built to explain the variability of OC photo-reactivity. The hydrophilicity of OC was largely influenced through the content of hydrophilic functional groups such as aromatic carbonyls (Ar–C=O) and OH, while the light absorption capacity and •OH generation ability of OC were determined by aromatic C–H (Ar–H) and Ar–C=O groups. The sulfate formation rate deriving from the photoreaction of SO2 with OC was estimated to be 0.43–1.33 µg m−3 h−1, suggesting an importance role of OC photochemistry as a key sulfate source.
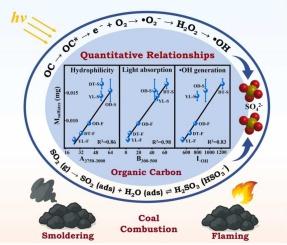
煤燃烧有机碳的理化性质与SO2非均相光氧化制硫酸盐的定量关系
煤的燃烧显著增加了大气中的有机碳(OC),它可以作为SO2转化为硫酸盐的光敏位点。光照能显著促进煤燃烧OC吸收SO2形成硫酸盐。这些OC具有可变的光反应性,表现为不同的SO2吸收系数和硫酸盐产量。证实了OC对SO2的光反应性很大程度上取决于其亲水性、光吸收能力和•OH生成能力等理化性质。这些性质与SO2吸收和硫酸盐生成的线性关系已经很好地解释了OC光反应性的变异性。OC的亲水性很大程度上受芳香羰基(Ar-C =O)和OH等亲水性官能团含量的影响,而OC的光吸收能力和生成•OH的能力则由芳香C-H (Ar-H)和Ar-C =O基团决定。SO2与OC的光化学反应生成硫酸盐的速率为0.43 ~ 1.33µg m−3 h−1,表明OC光化学是硫酸盐的重要来源。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


