Analysis of airway inflammation demonstrates a mechanism for T2-biologic failure in asthma
IF 11.2
1区 医学
Q1 ALLERGY
引用次数: 0
Abstract
Background
Targeted type 2 (T2) biologics have transformed asthma care, but the clinical response to biologic therapy varies between patients.
Objective
We sought to assess airways inflammation in T2-high asthmatic patients treated with anti–IL-5 biologics to investigate whether differential mechanism of airway inflammation explains varied response to biologics.
Methods
Proteomic analysis (Olink, 1463 protein panel) and high-sensitivity cytokine analysis (ELISAs) were performed on induced sputum from T2-high severe asthmatic patients in the UK multicenter Mepolizumab EXacerbation study. Samples included were pre-mepolizumab (n = 28), stable on mepolizumab (n = 43), and at first exacerbation (n = 26).
Results
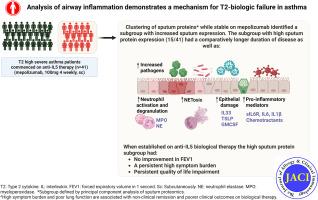
Clustering of sputum proteins while stable on mepolizumab identified 2 clusters. Cluster 1 had increased differentially expressed sputum proteins pre-mepolizumab, stable on mepolizumab, and at exacerbation. Patients in cluster 1 were younger at diagnosis, had a longer duration of asthma, lower FEV1%, and higher 5-Question Asthma Control Questionnaire score on mepolizumab. Cluster 1 had increased expression of proinflammatory cytokines (IL-1β, IL-6, and soluble IL-6R), epithelial alarmins (thymic stromal lymphopoietin [TSLP] and IL-33), and neutrophil activation (myeloperoxidase [MPO], neutrophil elastase [NE], and neutrophil extracellular trap concentration [NET]). All patients were T2-high with no difference in fractional exhaled nitric oxide, eosinophil number, or activity (eosinophil-derived neurotoxin, EDN) across the 2 clusters.
Conclusions
In a cohort of T2-high severe asthmatic patients, a subgroup of patients with long duration of disease had worse clinical parameters, increased sputum proteins with increased markers of neutrophil activity, proinflammatory cytokines, and epithelial alarmins even when stable on mepolizumab. This suggests the presence of biology not treated by targeted T2 biologics, which may contribute to poorer outcomes on biologics and could be a treatable airways trait in severe asthma.
气道炎症分析揭示了哮喘患者t2生物学功能衰竭的机制。
背景:靶向T2生物制剂已经改变了哮喘治疗,但不同患者对生物治疗的临床反应不同。目的评估接受抗il - 5生物制剂治疗的t2 -高哮喘患者的气道炎症,探讨气道炎症的不同机制是否解释了对生物制剂的不同反应。方法对英国多中心Mepolizumab加重(MEX)研究中t2值高的重症哮喘患者的诱导痰进行蛋白质组学分析(Olink®,1463蛋白面板)和高灵敏度细胞因子分析(elisa)。纳入的样本包括mepolizumab前(n=28), mepolizumab稳定(n= 43)和首次加重(n=26)。结果mepolizumab稳定的痰蛋白聚类鉴定出两个聚类。集群1在美泊珠单抗前差异表达的痰蛋白增加,在美泊珠单抗和恶化时稳定。第1组患者在诊断时较年轻,哮喘持续时间较长,mepolizumab治疗时FEV1%较低,ACQ5较高。集群1促炎细胞因子(IL1β, IL6, sIL6R),上皮警报器(TSLP, IL 33)和中性粒细胞活化(MPO, NE和中性粒细胞胞外陷阱(NET))的表达增加。所有患者均为t2高,两组患者的FeNO、嗜酸性粒细胞数量或活性(EDN)无差异。结论:在一组t2 -高的重症哮喘患者中,一组病程较长的患者临床参数较差,痰蛋白升高,中性粒细胞活性、促炎细胞因子和上皮报警标志物升高,即使在美波珠单抗稳定的情况下也是如此。这表明未被靶向t2生物制剂治疗的生物学存在可能导致生物制剂治疗效果较差,并且可能是严重哮喘可治疗的气道特征。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
25.90
自引率
7.70%
发文量
1302
审稿时长
38 days
期刊介绍:
The Journal of Allergy and Clinical Immunology is a prestigious publication that features groundbreaking research in the fields of Allergy, Asthma, and Immunology. This influential journal publishes high-impact research papers that explore various topics, including asthma, food allergy, allergic rhinitis, atopic dermatitis, primary immune deficiencies, occupational and environmental allergy, and other allergic and immunologic diseases. The articles not only report on clinical trials and mechanistic studies but also provide insights into novel therapies, underlying mechanisms, and important discoveries that contribute to our understanding of these diseases. By sharing this valuable information, the journal aims to enhance the diagnosis and management of patients in the future.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


