Optimization and stability evaluation of a pediatric spironolactone oral suspension: Formulation development and analytical validation
IF 4.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2025-06-26
DOI:10.1016/j.ejpb.2025.114801
引用次数: 0
Abstract
The pediatric population often faces challenges in accessing appropriate medication formulations, particularly for circumstances like congenital heart disease requiring spironolactone therapy. This study aimed to optimize the pharmaceutical formulation of oral suspension spironolactone for pediatric use and assess its stability. A formulation with 0.2 % xanthan gum in InOrpha® was developed, showing improved stability and reduced sedimentation. Analytical method validation confirmed accuracy and precision for spironolactone quantification, while forced degradation studies ensured stability-indicating capability. Stability assessments demonstrated the oral suspension’s chemical, physical, and microbiological stability for up to 135 days pre-bottle opening and 37 days post-opening under varied storage conditions. This study provides crucial insights into enhancing spironolactone formulation for pediatric patients. Further research is needed to assess pharmacokinetic parameters such as bioavailability and pharmacodynamics to fully ascertain its efficacy in pediatric populations.
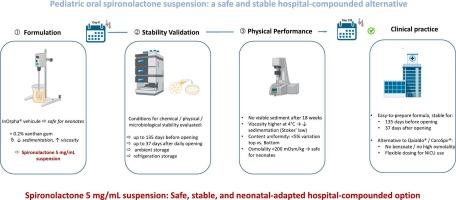
小儿螺内酯口服混悬液的优化和稳定性评价:配方开发和分析验证。
儿科人群在获得适当的药物配方方面经常面临挑战,特别是对于需要螺内酯治疗的先天性心脏病等情况。本研究旨在优化小儿口服螺内酯悬浮液的处方并评价其稳定性。开发了一种含有0.2 % InOrpha®黄原胶的配方,显示出更好的稳定性和减少沉淀。分析方法验证证实了螺内酯定量的准确性和精密度,而强制降解研究确保了稳定性指示能力。稳定性评估表明,在不同的储存条件下,口服混悬液的化学、物理和微生物稳定性可达135 天开瓶前和37 天开瓶后。这项研究为加强小儿患者的螺内酯制剂提供了至关重要的见解。需要进一步的研究来评估药代动力学参数,如生物利用度和药效学,以充分确定其在儿科人群中的疗效。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


