Optimized synthesis of aroyl-S,N-ketene acetals by omission of solubilizing alcohol cosolvents.
IF 2.1
4区 化学
Q2 CHEMISTRY, ORGANIC
Beilstein Journal of Organic Chemistry
Pub Date : 2025-06-20
eCollection Date: 2025-01-01
DOI:10.3762/bjoc.21.97
引用次数: 0
Abstract
Aroyl-S,N-ketene acetals are obtained by condensation of aroyl chlorides and 2-methyl-N-benzylbenzothiazolium salts in 1,4-dioxane at room temperature in short reaction time in 20-99% yield. This protocol represents a considerable improvement over the standard synthesis in 1,4-dioxane/ethanol mixtures at elevated temperatures.
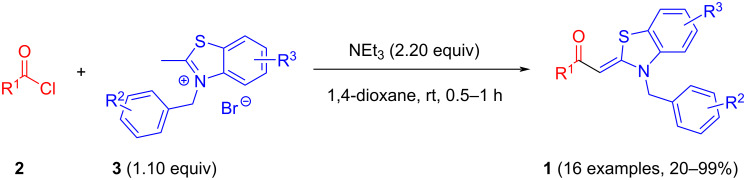
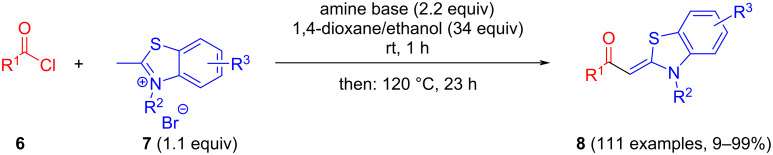
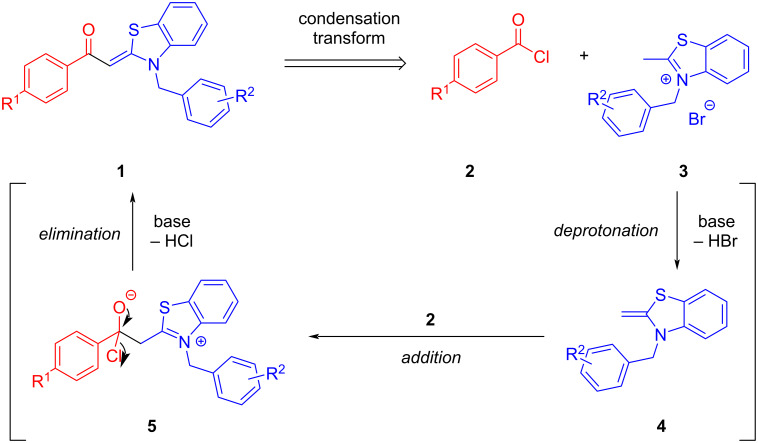
省略醇共溶剂,优化了芳烃- s, n -烯酮缩醛的合成。
在室温下,芳烃氯与2-甲基- n -苄基苯并噻唑盐在1,4-二恶烷中缩合,反应时间短,产率为20-99%,得到了芳烃- s, n -烯酮缩醛。该方案代表了在1,4-二恶烷/乙醇混合物中在高温下的标准合成的相当大的改进。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
4.90
自引率
3.70%
发文量
167
审稿时长
1.4 months
期刊介绍:
The Beilstein Journal of Organic Chemistry is an international, peer-reviewed, Open Access journal. It provides a unique platform for rapid publication without any charges (free for author and reader) – Platinum Open Access. The content is freely accessible 365 days a year to any user worldwide. Articles are available online immediately upon publication and are publicly archived in all major repositories. In addition, it provides a platform for publishing thematic issues (theme-based collections of articles) on topical issues in organic chemistry.
The journal publishes high quality research and reviews in all areas of organic chemistry, including organic synthesis, organic reactions, natural product chemistry, structural investigations, supramolecular chemistry and chemical biology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


