Decoding the [3 + 2] cycloaddition of a furan–imine oxide with styrene: mechanism, selectivity and bioactivity†
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
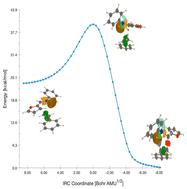
In this investigation, molecular electron density theory (MEDT) is used to study the [3 + 2] cycloaddition (32CA) reaction between 1-(furan-2-yl)-N-phenylmethanimine oxide () and styrene (). In fact, density functional theory (DFT) calculations at the M06-2X-D3/6-311G(d,p) level of theory in benzene at room temperature were performed to describe the regio- and stereoselectivity and the mechanism of the 32CA reaction studied. Using the CDFT approach, is a moderate electrophile and a strong nucleophile, while is classified as a moderate electrophile and a moderate nucleophile. As a consequence, the 32CA reaction under study is characterized by a non-polar characteristic. Analysis of thermodynamic data indicates that the compound is both thermodynamically and kinetically more favored than the other compounds, which aligns perfectly with the experimental results. BET analysis shows that this reaction takes place via a non-polar one-step cycloaddition with low asynchronous transition states. The QTAIM and ELF approaches further confirmed this asynchronous characteristic. Molecular docking analysis revealed that the product exhibited notable binding affinity to the 1CIN protease, highlighting its potential as a therapeutic inhibitor. Although drug-likeness evaluations confirmed compliance with Lipinski's rule of five, suggesting favorable pharmacokinetic properties, the PASS analysis indicated a limited range of predicted biological activities for the compound.
呋喃-氧化亚胺与苯乙烯的[3 + 2]环加成反应:机理、选择性和生物活性。
本研究采用分子电子密度理论(MEDT)研究了1-(呋喃-2-基)- n-苯基甲基氧化亚胺(1)与苯乙烯(2)之间的[3 + 2]环加成(32CA)反应。事实上,用M06-2X-D3/6-311G(d,p)水平的密度泛函数理论(DFT)计算了室温下苯中32CA反应的区域选择性和立体选择性及其机理。使用CDFT方法,1是一个中等亲电试剂和一个强亲核试剂,而2被分类为中等亲电试剂和中等亲核试剂。因此,所研究的32CA反应具有非极性特征。热力学数据分析表明,间端化合物在热力学和动力学上都比其他化合物更有利,这与实验结果完全一致。BET分析表明,该反应是通过非极性一步环加成进行的,具有低异步过渡态。QTAIM和ELF方法进一步证实了这种异步特性。分子对接分析显示,后端产物与1CIN蛋白酶具有显著的结合亲和力,突出了其作为治疗抑制剂的潜力。虽然药物相似性评估证实符合Lipinski的五法则,表明有利的药代动力学特性,但PASS分析表明该化合物的预测生物活性范围有限。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


