Faecal microbiota transplantation as a novel approach for autism-associated anxiety: A critical therapeutic appraisal
Q2 Medicine
引用次数: 0
Abstract
Autism Spectrum Disorder (ASD) is a complex neurodevelopmental condition characterized by social communication deficits and restrictive, repetitive behaviors. A significant proportion of individuals with ASD also suffer from anxiety disorders, further compounding their behavioral and emotional challenges. Conventional therapies for anxiety in ASD, including pharmacological and behavioral interventions, often yield suboptimal results and carry notable limitations. Growing research highlights the critical role of the gut-brain axis in neurodevelopment and emotional regulation, with gut microbiota dysbiosis increasingly implicated in both ASD and anxiety pathogenesis. Faecal microbiota transplantation (FMT), a therapeutic approach aimed at restoring microbial homeostasis by transferring fecal material from healthy donors, has emerged as a novel intervention of interest. Preclinical studies have demonstrated that alterations in gut microbiota can modulate social behaviors and anxiety-like symptoms, with FMT reversing many pathological features in animal models. Early clinical investigations, though limited, suggest that FMT may improve gastrointestinal health, core ASD symptoms, and comorbid anxiety. Mechanistically, FMT is thought to reduce neuroinflammation, restore neurotransmitter balance, and normalize stress responses by enhancing gut microbial diversity and metabolic function. However, significant challenges remain, including concerns about safety, standardization, donor selection, and regulatory approval. Future research must focus on large-scale, controlled trials and the identification of biomarkers predictive of FMT response to establish its therapeutic potential more conclusively. This review critically examines the existing evidence, explores the mechanistic pathways linking gut microbiota to anxiety in ASD, and discusses the future directions necessary to translate FMT into a viable clinical strategy for autism-associated anxiety.
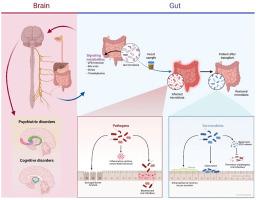
粪便微生物群移植作为一种治疗自闭症相关焦虑的新方法:一项关键的治疗评估
自闭症谱系障碍(ASD)是一种复杂的神经发育疾病,其特征是社会沟通缺陷和限制性、重复性行为。很大一部分ASD患者还患有焦虑症,这进一步加剧了他们的行为和情感挑战。ASD焦虑的传统治疗方法,包括药理学和行为干预,往往产生不理想的结果,并有明显的局限性。越来越多的研究强调了肠-脑轴在神经发育和情绪调节中的关键作用,肠道微生物群失调越来越多地与ASD和焦虑发病机制有关。粪便微生物群移植(FMT)是一种旨在通过转移健康捐赠者的粪便物质来恢复微生物稳态的治疗方法,已成为一种新的干预措施。临床前研究表明,肠道微生物群的改变可以调节社会行为和焦虑样症状,在动物模型中,FMT逆转了许多病理特征。早期临床调查虽然有限,但表明FMT可能改善胃肠道健康、核心ASD症状和共病焦虑。从机制上讲,FMT被认为通过增强肠道微生物多样性和代谢功能来减少神经炎症,恢复神经递质平衡,并使应激反应正常化。然而,重大挑战仍然存在,包括对安全性、标准化、供体选择和监管批准的担忧。未来的研究必须集中在大规模的对照试验和识别预测FMT反应的生物标志物上,以更确切地确定其治疗潜力。这篇综述对现有的证据进行了批判性的审查,探讨了将肠道微生物群与自闭症相关焦虑联系起来的机制途径,并讨论了将FMT转化为自闭症相关焦虑的可行临床策略所必需的未来方向。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Medicine in Microecology
Medicine-Gastroenterology
CiteScore
5.60
自引率
0.00%
发文量
16
审稿时长
76 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


