The long-term effects of chemotherapy on normal blood cells
IF 29
1区 生物学
Q1 GENETICS & HEREDITY
引用次数: 0
Abstract
Several chemotherapeutic agents act by increasing DNA damage in cancer cells, triggering cell death. However, there is limited understanding of the extent and long-term consequences of collateral DNA damage in normal tissues. To investigate the impact of chemotherapy on mutation burdens and the cell population structure of normal tissue, we sequenced blood cell genomes from 23 individuals aged 3–80 years who were treated with a range of chemotherapy regimens. Substantial additional somatic mutation loads with characteristic mutational signatures were imposed by some chemotherapeutic agents, but the effects were dependent on the drug and blood cell types. Chemotherapy induced premature changes in the cell population structure of normal blood, similar to those caused by normal aging. The results show the long-term biological consequences of cytotoxic agents to which a substantial fraction of the population is exposed as part of disease management, raising mechanistic questions and highlighting opportunities for the mitigation of adverse effects. Mutational signature analysis of blood cells isolated from 23 chemotherapy-exposed samples and 9 nonexposed controls characterizes the effects of various drugs on mutational burden, signature exposure and cell types.
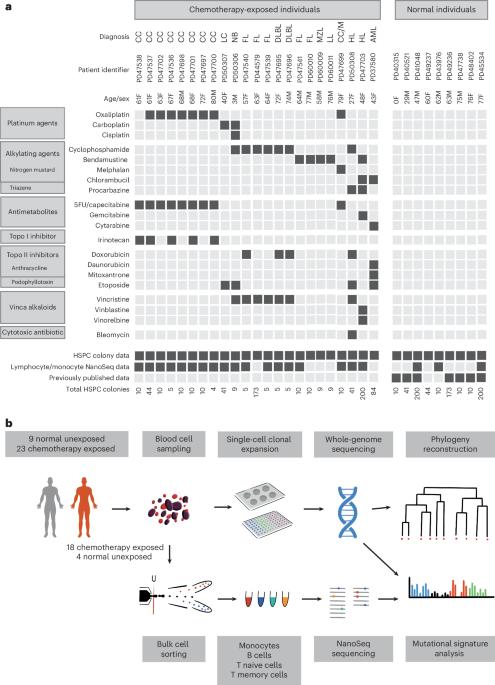

化疗对正常血细胞的长期影响
一些化疗药物的作用是增加癌细胞的DNA损伤,引发细胞死亡。然而,对正常组织中附带DNA损伤的程度和长期后果的了解有限。为了研究化疗对突变负担和正常组织细胞群结构的影响,我们对23名接受一系列化疗方案治疗的3-80岁患者的血细胞基因组进行了测序。一些化疗药物施加了具有特征性突变特征的大量额外体细胞突变负荷,但其影响取决于药物和血细胞类型。化疗引起了正常血液细胞群结构的过早变化,类似于正常衰老引起的变化。研究结果表明,作为疾病管理的一部分,有相当一部分人口暴露于细胞毒性制剂的长期生物学后果,提出了机制问题,并突出了减轻不利影响的机会。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature genetics
生物-遗传学
CiteScore
43.00
自引率
2.60%
发文量
241
审稿时长
3 months
期刊介绍:
Nature Genetics publishes the very highest quality research in genetics. It encompasses genetic and functional genomic studies on human and plant traits and on other model organisms. Current emphasis is on the genetic basis for common and complex diseases and on the functional mechanism, architecture and evolution of gene networks, studied by experimental perturbation.
Integrative genetic topics comprise, but are not limited to:
-Genes in the pathology of human disease
-Molecular analysis of simple and complex genetic traits
-Cancer genetics
-Agricultural genomics
-Developmental genetics
-Regulatory variation in gene expression
-Strategies and technologies for extracting function from genomic data
-Pharmacological genomics
-Genome evolution
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


