Combined intestinal transcriptome and microbiome profiling highlights microbiota involved in growth and immunity of the Reeves’ turtle (Mauremys reevesii)
IF 4.4
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
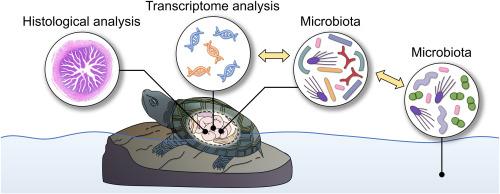
The intestine serves as the primary digestive and essential mucosal immune organ, harboring diverse microbiota that play crucial roles in digestion, absorption, and host immunity. However, the association between the intestinal microbiota and host growth and immunity remains insufficiently established, particularly in ectothermic animals where microbiota are easily influenced by external environments. Herein, a comparative analysis of histology, gene expression, transcriptome, and microbiome was deployed to systematically investigate the potential relevance of growth traits, immune responses, and gut microbiota between Reeves' turtles with inferior (IGP) or superior growth performance (SGP). Our results revealed that, in comparison to the IGP group, the SGP group exhibited histological intestinal structures that were more conducive to digestion and absorption, thereby aligning with its superior growth traits. Moreover, transcriptomic annotation identified 50 differentially expressed genes (DEGs) associated with growth and 70 DEGs involved in immunity. These DEGs showed significant enrichment in growth- and immunity-related GO terms and KEGG pathways. Further RT-PCR analyses validated the expression profiles of several genes related to growth and immunity across multiple tissues. In addition, analysis of microbial abundance revealed the presence of various sensitive indicator genera, such as Lactobacillus and Brevundimonas, which potentially imply host growth performance and immune response. At the phylum level, a more diverse and stable microbial interaction between intestinal microbiota in the SGP group and aquaculture water was predicted to contribute to superior growth performance and immunity. Furthermore, correlation analyses revealed a potential interplay between indicator genera with DEGs associated with growth and immune responses. Taken together, these findings highlight the intricate interplay of intestinal microbiota, environmental microbiota, as well as growth- and immunity-related genes in shaping host traits, providing valuable insights into promoting healthy culture practices for Reeves’ turtles from both molecular and microbiological perspectives.
联合肠道转录组和微生物组分析强调了里氏龟生长和免疫中涉及的微生物群(Mauremys reevesii)
肠道是主要的消化和必不可少的粘膜免疫器官,拥有多种微生物群,在消化、吸收和宿主免疫中起着至关重要的作用。然而,肠道微生物群与宿主生长和免疫之间的关系仍然没有充分确定,特别是在微生物群容易受到外部环境影响的恒温动物中。本文通过对组织学、基因表达、转录组和微生物组的比较分析,系统地研究了生长性能较差(IGP)和生长性能较优(SGP)的里夫斯龟的生长性状、免疫反应和肠道微生物群之间的潜在相关性。我们的研究结果显示,与IGP组相比,SGP组表现出更有利于消化和吸收的肠道组织结构,从而与其优越的生长性状相一致。此外,转录组注释鉴定了50个与生长相关的差异表达基因(deg)和70个与免疫相关的差异表达基因(deg)。这些deg在生长和免疫相关的GO术语和KEGG途径中显示出显著的富集。进一步的RT-PCR分析验证了多个组织中与生长和免疫相关的几个基因的表达谱。此外,微生物丰度分析显示存在多种敏感指示属,如乳酸菌和Brevundimonas,可能影响宿主的生长性能和免疫反应。在门水平上,预计SGP组肠道菌群与养殖水体之间的微生物相互作用更加多样化和稳定,有助于提高生长性能和免疫力。此外,相关分析揭示了与生长和免疫应答相关的deg指标属之间的潜在相互作用。综上所述,这些发现突出了肠道微生物群、环境微生物群以及生长和免疫相关基因在塑造宿主性状中的复杂相互作用,从分子和微生物的角度为促进里夫斯龟的健康培养实践提供了有价值的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


