High-performance azithromycin delivery via chitosan-tryptophan modified polyethersulfone transdermal membranes
IF 6.5
Q1 CHEMISTRY, APPLIED
Carbohydrate Polymer Technologies and Applications
Pub Date : 2025-06-24
DOI:10.1016/j.carpta.2025.100911
引用次数: 0
Abstract
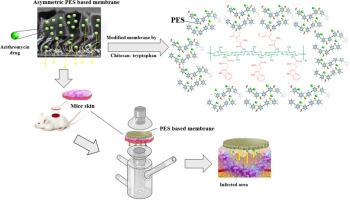
This study investigates a novel asymmetric polyethersulfone (PES) membrane incorporated with chitosan-tryptophan (CS-W) for enhanced azithromycin delivery in vitro and ex vivo. Key fabrication parameters, including drug concentration (500 mg/L), membrane thickness (300 μm), modifier percentage (1.5 %), polymer percentage (17 %), and pore maker content (2 %), were optimized to improve membrane performance.
The optimized CS-W membrane (M4) achieved a drug release of 407 mg/L, compared to 240 mg/L for the unmodified membrane (M1), using a 500 mg/L azithromycin solution. Cell viability reached ∼80 %, and hemolysis was ∼4.6 %, confirming biocompatibility. The water vapor transmission rate increased by 9.25 %, supporting enhanced moisture handling. Long-term testing confirmed membrane reusability. Also the evaluations showed acceptable antibacterial activity.
The improved performance results from the membrane’s asymmetric structure: a dense top layer ensures sustained release, while a porous sub-layer acts as a drug reservoir. The drug release followed a zero-order kinetic model, making the membrane a promising candidate for sustained drug delivery applications.
壳聚糖-色氨酸改性聚醚砜透皮膜高效递送阿奇霉素
本文研究了一种新型不对称聚醚砜(PES)膜与壳聚糖-色氨酸(CS-W)的结合,以增强阿奇霉素的体外和体外递送。通过优化药物浓度(500 mg/L)、膜厚度(300 μm)、改性剂含量(1.5%)、聚合物含量(17%)、造孔剂含量(2%)等关键制备参数,提高了膜的性能。在500 mg/L的阿奇霉素溶液中,优化后的CS-W膜(M4)的药物释放量为407 mg/L,而未修饰的膜(M1)的药物释放量为240 mg/L。细胞存活率达到~ 80%,溶血率达到~ 4.6%,证实了生物相容性。水蒸气透过率增加了9.25%,支持增强的水分处理。长期测试证实了膜的可重复使用性。结果表明,抗菌活性良好。这种性能的提高源于膜的不对称结构:致密的顶层确保持续释放,而多孔的下层则充当药物储存库。药物释放遵循零级动力学模型,使膜成为持续药物递送应用的有希望的候选者。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


