Identification of residues mediating homotropic regulation in human hexokinase 3 reveals a common allosteric interface shared with hexokinase 1
IF 3
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Vertebrate hexokinases (HKs) display a variety of allosteric phenomena, including activation and inhibition by both homotropic and heterotropic ligands. The extent to which these homologs share a common allosteric mechanism is unknown. A unique trait of the vertebrate hexokinase 3 (HK3) orthologs is substrate inhibition by glucose. Here, we demonstrate that the isolated, regulatory N-terminal domain of human HK3 contains a low affinity glucose binding site whose dissociation constant (3.2 ± 0.4 mM) approximates the Ki value for glucose (11 ± 2 mM) observed in assays of the full-length enzyme. The isolated, catalytic C-terminal domain harbors a high affinity glucose binding site whose dissociation constant (6.2 ± 0.6 μM) resembles the Km value for glucose (53 ± 1 μM). Substitution of Asn221 in full-length HK3, which lies within the N-terminal glucose binding site, reduces substrate inhibition by 30-fold while leaving other steady-state kinetic parameters unchanged. Homotropic inhibition of HK3 is largely independent of ATP concentrations, in contrast to heterotropic inhibition of hexokinase 1 (HK1) by glucose 6-phosphate, which is competitive with respect to ATP. Adding a 10-fold molar excess of the N-terminal domain to the C-terminal domain fails to alter substrate inhibition, suggesting that interdomain communication in HK3 requires their physical connection. Disrupting a coulombic interaction between N-terminal residue Asp264 and C-terminal residue Arg807, two conserved residues previously shown to participate in HK1 regulation, attenuates glucose inhibition of HK3. Our data support a common allosteric interface in HK1 and HK3, wherein effector binding at spatially distinct sites within the regulatory N-terminus is communicated to the catalytic C-terminus via conserved coulombic residues.
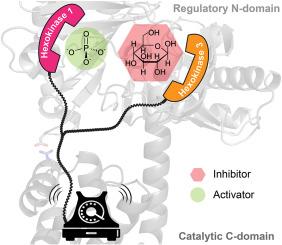
介导人己糖激酶3同向调节的残基鉴定揭示了与己糖激酶1共有的变构界面。
脊椎动物己糖激酶(HKs)表现出多种变构现象,包括同向和异向配体的激活和抑制。这些同源物在多大程度上共享一个共同的变构机制是未知的。脊椎动物己糖激酶3 (HK3)同源物的一个独特特征是葡萄糖对底物的抑制。在这里,我们证明了分离的人类HK3的调节n端结构域包含一个低亲和力的葡萄糖结合位点,其解离常数(3.2±0.4 mM)接近在全长酶的测定中观察到的葡萄糖的Ki值(11±2mm)。分离得到的催化c端结构域具有高亲和力的葡萄糖结合位点,其解离常数(6.2±0.6 μM)与葡萄糖的Km值(53±1 μM)相似。Asn221在位于n端葡萄糖结合位点的全长HK3上的替代,将底物抑制降低了30倍,同时保持其他稳态动力学参数不变。HK3的同向抑制在很大程度上与ATP浓度无关,而6-磷酸葡萄糖对HK1的异向抑制则与ATP竞争。向c端结构域添加10倍摩尔量的过量n端结构域并不能改变底物抑制,这表明HK3结构域间的通信需要它们的物理连接。破坏n端残基Asp264和c端残基Arg807之间的库伦相互作用,减弱了HK3的葡萄糖抑制作用,这两个保守残基先前被证明参与HK1调控。我们的数据支持HK1和HK3中一个共同的变弹性界面,其中调节n端内空间不同位置的效应结合通过保守的库仑残基传递到催化c端。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Archives of biochemistry and biophysics
生物-生化与分子生物学
CiteScore
7.40
自引率
0.00%
发文量
245
审稿时长
26 days
期刊介绍:
Archives of Biochemistry and Biophysics publishes quality original articles and reviews in the developing areas of biochemistry and biophysics.
Research Areas Include:
• Enzyme and protein structure, function, regulation. Folding, turnover, and post-translational processing
• Biological oxidations, free radical reactions, redox signaling, oxygenases, P450 reactions
• Signal transduction, receptors, membrane transport, intracellular signals. Cellular and integrated metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


