Inhibition of neuronal necroptosis via disruption of RIPK1-RIPK3 Interactions: The role of neural stem cell-derived exosomes in spinal cord injury recovery
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Spinal cord injury (SCI) poses a significant economic and public health burden. Exosomes derived from neural stem cells (NSC-Exos) are emerging as a promising therapeutic strategy for SCI repair, overcoming several limitations associated with both autologous and allogeneic neural stem cell therapies. This study demonstrates that NSC-Exos are efficiently internalized by the injured spinal cord after co-injection, resulting in substantial motor function recovery in murine models. Additionally, NSC-Exos effectively limit the expansion of the injury site, reduce neuronal degeneration, and attenuate neuroinflammatory responses. Notably, this is the first study to identify necroptosis as a novel therapeutic target for NSC-Exos in SCI recovery. We show that NSC-Exos inhibit neuronal necroptosis both in vivo and in vitro by disrupting the RIPK1-RIPK3 interaction, thereby preventing necrosome assembly. Furthermore, comprehensive transcriptomic analysis reveals that the ubiquitin-mediated proteolysis (UPS) pathway plays a crucial role in this process, a finding supported by experimental inhibition of ubiquitination. In conclusion, this study highlights the therapeutic potential of NSC-Exos in SCI treatment, particularly through the inhibition of necroptosis via disruption of the RIPK1-RIPK3 interaction, potentially involving UPS activation. These findings provide a foundation for future investigations into the molecular mechanisms underlying SCI recovery.
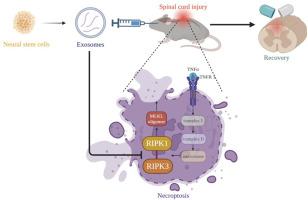
通过破坏RIPK1-RIPK3相互作用抑制神经元坏死:神经干细胞来源的外泌体在脊髓损伤恢复中的作用
脊髓损伤(SCI)造成了巨大的经济和公共卫生负担。神经干细胞衍生的外泌体(NSC-Exos)克服了自体和异体神经干细胞治疗的一些局限性,成为一种有前景的脊髓损伤修复治疗策略。本研究表明,nscs - exos在共注射后被损伤的脊髓有效内化,导致小鼠模型的运动功能大幅恢复。此外,NSC-Exos有效地限制了损伤部位的扩张,减少了神经元变性,并减轻了神经炎症反应。值得注意的是,这是第一个确定坏死性下垂作为NSC-Exos在SCI恢复中的新治疗靶点的研究。我们发现NSC-Exos通过破坏RIPK1-RIPK3相互作用,从而阻止坏死体的组装,从而在体内和体外抑制神经元坏死。此外,综合转录组学分析表明,泛素介导的蛋白水解(UPS)途径在这一过程中起着至关重要的作用,这一发现得到了泛素化抑制实验的支持。总之,本研究强调了NSC-Exos在SCI治疗中的治疗潜力,特别是通过破坏RIPK1-RIPK3相互作用来抑制坏死性下垂,可能涉及UPS激活。这些发现为进一步研究脊髓损伤恢复的分子机制奠定了基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


